Translate this page into:
Cutaneous non-tuberculous mycobacterial infections: An update
*Corresponding author: Mamatha George, Department of Dermatology, Malabar Medical College Hospital and Research Centre, Kozhikode, Kerala, India. tammu77@yahoo.com
-
Received: ,
Accepted: ,
How to cite this article: George M. Cutaneous non-tuberculous mycobacterial infections: An update. J Skin Sex Transm Dis 2023;5:90-7.
Abstract
Non-tuberculous mycobacteria (NTM) are increasingly recognized as causes of skin and soft-tissue infections. They include rapid-growing and slow-growing species. Hospital outbreaks related to contaminated water and in association with surgical and cosmetic procedures have been described. Infections are also associated with immunosuppression. NTM infections have a wide spectrum of clinical manifestations, though Mycobacterium marinum and Mycobacterium ulcerans manifest characteristic lesions – swimming pool granuloma and Buruli ulcer, respectively. NTM infection should be suspected when the skin infection (especially those following trauma or invasive procedure or in a patient with immunosuppression) does not respond to antibiotics. NTM are acid fast, but will be negative on cartridge based nucleic acid amplification tests for Mycobacterium tuberculosis. Diagnosis is confirmed by polymerase chain reaction test which is the gold standard. NTM show variable susceptibility to antimicrobials and no clear treatment guidelines are available. Surgical treatment may also be needed in some cases.
Keywords
Non-tuberculous mycobacteria
Atypical mycobacteria
Mycobacteria
Skin
INTRODUCTION
The term “Non-tuberculous mycobacteria” (NTM) refers to the group of all mycobacterial species other than Mycobacterium tuberculosis (M. tuberculosis) complex and M. leprae.[1] NTM are also referred to as atypical mycobacteria, environmental mycobacteria or mycobacteria other than tuberculosis. They are free-living, slender, non-motile, and acid-fast bacilli, that are ubiquitous in the environment, and have been isolated from water, soil, food products, plants, and domestic and wild animals including fish.[2,3] NTM are facultative, intracellular agents in humans and may be present as colonizers or as pathogens.
Skin and soft tissue infections (SSTI) caused by NTM are difficult to diagnose due to their wide spectrum of clinical presentations, as well as non-specific histopathology findings. Tissue culture and polymerase chain reaction (PCR) assays may be needed for diagnosis. Treatment is challenging and requires multiple antibiotic combinations and surgical intervention depending on the causative organism.[3]
NTM are traditionally classified based on the rate of growth in vitro as rapid-growing mycobacteria (RGM) and slow-growing mycobacteria (SGM).[4] They are further classified based on pigment production following exposure to light.[5] RGM grow rapidly in culture and hence can be isolated within 7 days.[4,5]
For some RGM species including M. fortuitum, M. abscessus, and M. chelonae, SSTIs are the most common presentation. Of the SGM species, Mycobacterium avium complex (MAC: M. avium, M. intracellulare, M. chimaera), M. ulcerans (Buruli ulcer), M. marinum (fish-tank granuloma), M. haemophilum, and a few other rarer species have been implicated in SSTI and are being increasingly reported in recent years.[2] While these are the most common agents, virtually any species of NTM can cause cutaneous disease. NTM infection should be considered in all patients with treatment resistant SSTI.[2]
EPIDEMIOLOGY AND MODES OF TRANSMISSION
NTM are generally acquired through environmental exposure and there had been no reports of human-to-human transmission, until a case of potential M. abscessus transmission between cystic fibrosis patients was reported in 2013.[6]
Skin infection by NTM typically occurs following minor trauma, especially accidental inoculation such as by needles, wood splints, or fish spines. They are also reported to occur by accidental contamination of surgical or open wounds – and have been associated with nosocomial outbreaks following surgical or cosmetic procedures. Contaminated water has been identified as the source in many of these outbreaks.
Mycobacterial species are frequently found in hospital environment because of their biofilm forming capacity, making them highly resistant to standard decontamination techniques.[7]
Being relatively resistant to standard disinfectants such as chlorhexidine, glutaraldehyde, alcohol, and formaldehyde, RGM species are more commonly associated with nosocomial outbreaks.[3,8] Rarely, infection may spread hematogenously in immunocompromised hosts.[7]
The incidence of NTM infection has increased dramatically over the past several years. The incidence of cutaneous NTM infections in 2000–2009 was nearly threefold higher than in 1980–1999. This is due to the increase in procedures such as tattooing, mesotherapy, liposuction, dermal fillers, and body piercing.[9] Clustered cases have been reported from non-hospital settings such as tattoo parlors, nail salons, fish markets, and acupuncture centers.[7] Improved detection by rapid and reliable molecular methods has also contributed to the increased recognition and identification of NTM.
RISK FACTORS
Immunocompromised status due to human immunodeficiency virus infection, organ transplantation, and biological therapy using tumor necrosis factor-α (TNF-α) inhibitors predisposes to disseminated NTM infection. Individuals with Mendelian susceptibility to mycobacterial disease have selective susceptibility to clinical disease caused by environmental mycobacteria.[10]
CUTANEOUS MANIFESTATIONS
SSTI by NTM have highly variable manifestations, which can contribute to a delay in diagnosis. Clinically lesions may appear as papules, plaques, nodules, abscesses, sinuses, ulcers, panniculitis, folliculitis, and cellulitis. Infection may present in a sporotrichoid pattern, tracking along lymphatics from the site of inoculation, particularly in M. marinum infection. M. ulcerans and M. marinum produce characteristic clinical pictures (see below).[3,7,11] Reactive manifestations due to disseminated NTM have been described and include Sweet’s syndrome, generalized pustulosis, erythema nodosum, and pustular psoriasis.[3]
Rapidly growing NTM species
The three primary SSTI-causing RGM species are M. abscessus, M. chelonae, and M. fortuitum. Contaminated water is a frequent source of infection. They are increasingly found in hospital settings following trauma, surgery, and cosmetic procedures. The clinical presentation is varied.
M. abscessus
Among the RGM species, M. abscessus is the major cause of skin infections. It was first described by Moore and Frerichs in 1953 in a woman with chronic osteoarthritis of knee, who developed a gluteal abscess.[12] M. abscessus is the most pathogenic and clinically challenging of RGM species. It frequently demonstrates a higher degree of antimicrobial resistance. The single most important factor that determines the course and prognosis of M. abscessus infection is the underlying immune status of the host.[13] Disseminated cutaneous infections have been reported in patients on immunosuppressive therapy.[14]
Multiple outbreaks following medical, surgical, and cosmetic procedures and acupuncture have been reported.[1] Forty-five surgical site infections due to M. abscessus among pediatric patients were reported in 1998 from New Delhi, India, in a single hospital.[15] M. abscessus was isolated from the tap water in the operating rooms, which was used for rinsing instruments. In 2004, the Centers for Disease Control and Prevention reported an outbreak of 24 cases of M. abscessus infection among patients who had undergone a variety of cosmetic procedures in The Dominican Republic.[16]
Cutaneous manifestations are non-specific. Initial presentation may be an abscess at the site of inoculation. Nodules, abscesses, and non-healing ulcers may be seen [Figure 1]. Sporotrichoid distribution of lesions has also been reported.[17] Disseminated disease presents with systemic symptoms and may be associated with red to violaceous, subcutaneous nodules, and lymphadenopathy.[18,19]

-
Mycobacterium abscessus infection on the scalp of a patient following hair transplantation presenting as multiple nodular lesions and abscesses – picture courtesy Dr. Anisha K Janardhanan, Consultant Dermatologist, Baby Memorial Hospital, Kozhikode.
M. chelonae
M. chelonae can infect surgical and traumatic wounds, and cause injection site abscess, especially in diabetic patients on insulin. Reported infections with M. chelonae are on the rise.[20] Surgical site infections as well as wound infections are associated with tap water contamination – such an outbreak was reported from India in association with laparoscopic surgery [Figure 2].[21] Infection is also reported following acupuncture, tattooing, mesotherapy, and cosmetic procedures.

-
Mycobacterium chelonae infection presenting as nodules with discharging sinuses at the laproscopic port site on the abdomen – picture courtesy Dr Soumya Jagadeesan, Associate Professor, Dept of Dermatology, Amrita Institute of Medical sciences, Kochi.
There are reports of non-healing leg ulcer after penetrating injury.[22] Fatal and disseminated chelonae infections have been rarely reported in immunosuppressed patients and following administration of biologicals such as adalimumab.[2,23]
Cutaneous manifestations include localized cellulitis, nodules, sinuses, abscesses, and ulcers.[1]
They are resistant to conventional anti-tuberculosis treatment (ATT), with varied susceptibility to other antimicrobials.
M. fortuitum
M. fortuitum was first isolated by Da Costa Cruz in 1938, from a patient with an injection site abscess.[24,25] In contrast to M. chelonae and M. abscessus infections, which commonly occur in immunocompromised hosts, infection due to M. fortuitum tends to occur mostly in otherwise healthy individuals of younger age group, and typically present with single lesion at the site of trauma.[1,26] In 2002, Winthrop et al. reported a community outbreak of M. fortuitum furunculosis following footbath at a nail salon in California. Shaving the legs before the procedure was identified as a possible risk factor.[27] Thus, mycobacterial infections should be considered and enquiry should be made regarding recent pedicures underwent by the patient when lower extremity, treatment-resistant furunculosis is encountered.[26]
Lesions can vary from painful nodules, abscesses, ulcers, draining sinuses, and tracts to cellulitis. M. fortuitum is a rare cause of dialysis catheter line infection.[28]
M. fortuitum is more susceptible to antibiotics, adjuvant surgical intervention is recommended as and when necessary.
Slow growing NTM species
M. marinum (M. balnei)
M. marinum was first isolated by Aronso in 1926; later in 1951, Norden and Linel identified it as the pathogen in a patient, who developed a granulomatous lesion after visiting a contaminated swimming pool.[29,30] It is an aerobic organism which grows best at a temperature of 30–32°C, and poorly at 37°C.
M. marinum infection may be an occupational hazard, as in pet shop workers, or in fish fanciers with home aquariums as it is found both in fresh and salt water. It causes a characteristic skin infection termed fish-tank or swimming pool granuloma, which presents as solitary or multiple violaceous papulonodular lesions at the site of inoculation after 2–3 weeks, usually in the extremities. This may spread proximally in a sporotrichoid fashion due to lymphocutaneous spread or evolve to nodular, psoriasiform, or verrucous plaques which may later ulcerate [Figure 3]. Lymphadenopathy is rarely seen. Specific membrane lipids, called phenolic glycolipids, have been demonstrated to recruit macrophages to the site of infection, which facilitates further spread.[7]

-
Mycobacterium marinum infection presenting as nodules on the hand – picture courtesy Dr Soumya Jagadeesan, Associate Professor, Department of Dermatology, Amrita Institute of Medical sciences, Kochi.
Spontaneous resolution can occur after many months, while deeper infections can lead to tenosynovitis (“fish tank finger”), bursitis, arthritis, and osteitis.[31,32]
There are reports of outbreak of infection due to NTM, probably M. marinum in Pacific islanders (locally known as spam disease), which manifest as verrucous and keloid plaques.[33,34]
M. marinum infection associated with TNF-α inhibitors was first reported in 1994, in a patient, who developed septic arthritis following etanercept therapy. Similar infections have been reported following infliximab and adalimumab.[35-39]
M. ulcerans
Infection with this species was first described in 1948 by MacCallum et al. from Australia in patients presenting with solitary ulcerative lesions in extremities, and the Mycobacterium was named “Bairnsdale bacillus,” after the region, where most of the patients lived.[40] Later, it was renamed as M. ulcerans. Skin ulcers caused by M. ulcerans were reported from Buruli in Uganda in 1961, a region near the Nile River, and following several reports from the region, the disease was subsequently named Buruli ulcer (BU).[40]
M. ulcerans is the third most common cause of mycobacterial infection after tuberculosis and leprosy worldwide and BU is one of the skin-related neglected tropical diseases according to the WHO.[1]
Children and young adults are most commonly affected. Onset is typically as a subcutaneous nodule, which subsequently enlarges and ulcerates. It may later bore through the soft tissue, right up to the bone. Extremities are commonly affected, although head, neck, trunk, and genital region can be involved. Mycolactone, a highly diffusible and cytotoxic lipid has been identified as the pathogenic factor, and explains the distinct clinical presentation. Three major biological properties of mycolactone are cytotoxicity, immunosuppression, and analgesia, which correspond well to the characteristic features of the disease: extensive deep ulceration with thick yellowish necrotic tissue, undermining with limited inflammatory response and minimal or absent pain.[40-42]
Antibiotic therapy is usually effective; however, patients with severe illness or delayed initiation of treatment may have permanent deformities. Without treatment, the disease resolves in some patients, while in others it ends in contractures that cause disfigurement, long-term disability, and social stigmatization, known as “bankruptcy wound”.[41] Early detection and treatment is the only measure to prevent deleterious outcomes.[7,42]
MAC
This includes M. avium, M. intracellulare, M. chimaera, and other species. Primary cutaneous infections are rare. Skin lesions in disseminated disease have been described in immunocompromised patients, especially advanced AIDS. With the onset of the AIDS epidemic, MAC became more recognized as an opportunistic pathogen. Clinical manifestations include scaly papules, granulomatous plaques, subcutaneous nodules, pustules, verrucous ulcers, abscesses, and discharging sinuses.[3,43-45] A case of papulonecrotic tuberculid like presentation secondary to disseminated MAC infection in AIDS has been reported.[46]
Emerging pathogen: M. chimaera
M. chimaera was described by Tortoli et al. in 2004 as a colonizer of respiratory tract, in specimens from patients with chronic obstructive pulmonary disease and other lung diseases.[47] It is grouped under MAC. Since it shares features with M. avium, M. intracellulare and M. scrofulaceum, it was named M. chimaera – after chimera, a Greek mythological character composed of the parts of more than one animal.[47]
Clinical infections have a long and variable incubation period of up to 6 years. Outbreaks have been reported after open heart surgery. Cutaneous infection with M. chimaera is extremely rare. A case of cutaneous infection with M. chimaera was reported by Moutsoglou et al. in a patient with a non-healing leg ulcer.[48] George et al. reported a case of primary cutaneous infection with M. chimaera in a patient with diabetes mellitus that manifested with ulcerating nodules on the face [Figure 4].[49]

-
Mycobacterium chimaera infection presenting as ulcerating nodules on the face.
M. haemophilum
M. haemophilum is an aerobic fastidious organism, similar to M. marinum and M. ulcerans, and shows a predilection for the extremities, particularly over the joints. The name originates from the requirement of iron or hemin supplementation for growth.[50]
Infection generally occurs in immunocompromised hosts and may include erythematous to violaceous papules, plaques, or nodules which may progress to abscesses or deep-seated ulcers. It has certain similarities with M. leprae, including the presence of large quantities of docosanoic acid and analogous phenolic glycolipid. Immune reconstitution events similar to lepra reactions are described after initiation of anti-mycobacterial therapy.[44]
M. kansasii
M. kansasii is closely related antigenically to M. tuberculosis. Cutaneous infections may present as nodules, pustules, verrucous plaques, ulcers, and abscesses. The lesions may be arranged in a sporotrichoid pattern.[44] Similar to M tuberculosis, M.kansasii is present in nasopharyngeal secretions and can lead to periorificial cutaneous infection.
MICROBIOLOGICAL DIAGNOSIS
Tissue biopsy is the most sensitive means of obtaining a specimen.[3,44] Drainage material may also yield the organism. Specimens have to be initially analyzed with acid fast stain (e.g., Ziehl-Neelsen stain), cartridge-based nucleic acid amplification test (CB-NAAT - e.g., GeneXpert®) and culture. Culture is currently performed in liquid medium, in mycobacterial growth indicator tubes (MGIT – e.g., BACTEC®) which offer faster and more reliable detection compared to solid medium (Lowenstein-Jensen medium).[3,44]
All NTM are acid fast, hence will stain positively; however, the sensitivity is low. CB-NAAT is specific for M. tuberculosis complex, and hence will be negative for NTM. This combination of positive acid-fast stain and negative CBNAAT indicates the presence of NTM species.[3,44]
RGM species grow in culture within 7–10 days, while SGM species generally take more than 2 weeks. Once culture is positive, species identification is carried out on the growth using biochemical tests or PCR. Biochemical tests are hazardous and hence are not routinely done at present. 16S ribosomal ribonucleic acid (rRNA) gene analysis with PCR gives reliable identification of all known NTM species and is the standard. Some other targets for PCR are the rpoB gene (which has higher discriminatory power for RGM species) and hsp65 gene.[51] Amplification of the target deoxyribonucleic acid followed by denaturing high-performance liquid chromatography is another promising approach. Matrix-assisted laser desorption ionization time-of-flight mass spectrometry is a novel non-PCR method, where different species are identified by their unique spectral fingerprint.[3,44,51]
The final step would be testing for drug susceptibility, which is recommended especially for RGM species, which are tested for susceptibility to macrolides, quinolones, tetracyclines, and imipenem using microbroth dilution testing [Flow Chart 1].[3,44]
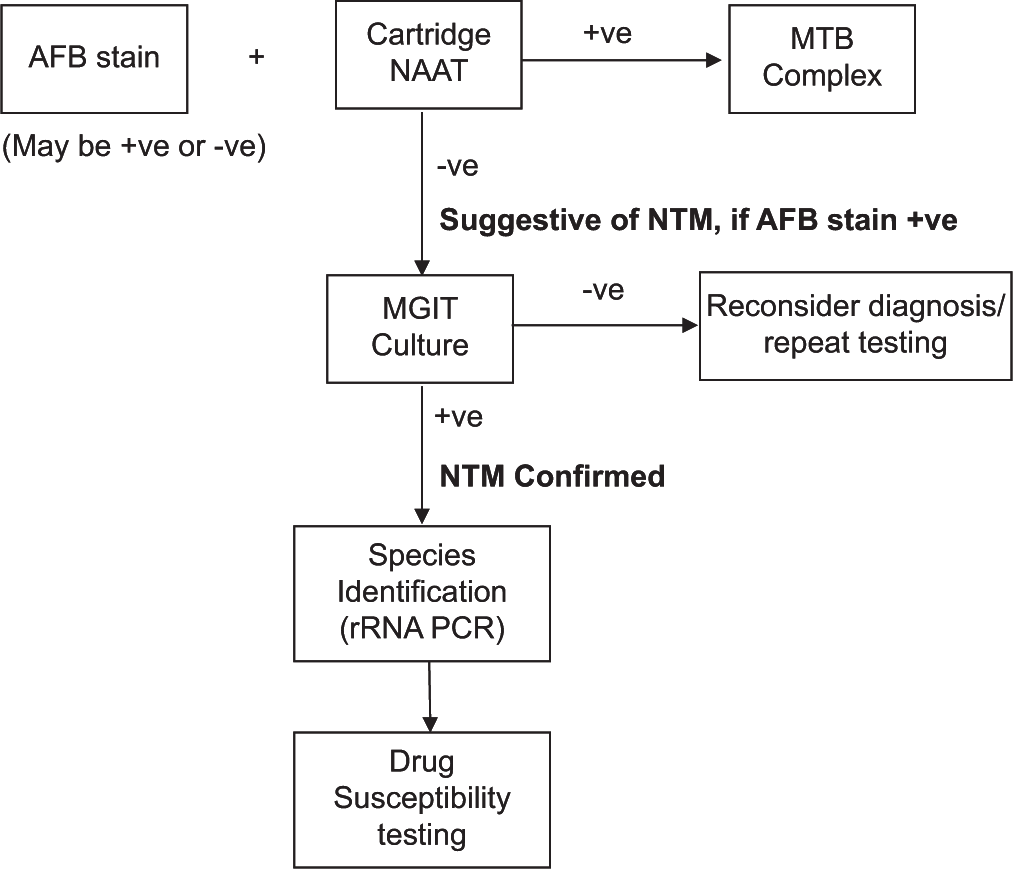
- Microbiological diagnosis of non-tuberculous mycobacteria. AFB: Acid fast bacillus, NAAT: Nucleic acid amplification test, MTB: Mycobacterium tuberculosis, NTM: Non-tuberculous mycobacteria, MGIT: Mycobacterial growth indicator tube, rRNA: ribosomal ribonucleic acid, PCR: Polymerase chain reaction.
HISTOPATHOLOGY
The histopathological features vary from an acute suppurative process with neutrophilic infiltration to typical granulomatous picture and macrophage infiltration. However, these are not specific to any species.[3] Combination of poorly formed granulomas and chronic suppuration should raise suspicion of NTM infection. Suppurative granuloma forming a central neutrophilic abscess has been described as a characteristic finding by Bartralot et al.[52] RGM species are more likely to cause suppurative inflammation and micro abscesses rather than granulomata. M. ulcerans infection is characterized by extensive necrosis and calcification, minimal inflammatory response, and occasional granulomas with large clusters of AFB.[53] M. marinum shows prominent epidermal changes such as acanthosis, pseudoepitheliomatous hyperplasia, and exocytosis.[54]
TREATMENT
Recommended treatment for the various NTM species is given in Table 1.[2,7,41,55-57]
| Organism | Preferred antimicrobials | Comments |
|---|---|---|
| RGM Species | Need susceptibility testing. 4–6 months therapy with at least two agents with in vitro activity. |
|
| M. fortuitum | Macrolides, quinolones, doxycycline, minocycline, amikacin, sulfonamides, cefoxitin, and imipenem | |
| M. chelonae | Tobramycin, clarithromycin, linezolid, imipenem, amikacin, clofazimine, doxycycline, and ciprofloxacin | Tobramycin more active than amikacin. Clarithromycin is the preferred second agent |
| M. abscessus | Macrolide and a parenteral agent such as amikacin, cefoxitin, imipenem or tigecycline, or combination of parenteral agents | Surgical resection often required |
| SGM Species | Delamanid and bedaquiline, tedizolid and avibactam are newer agents for SGM species | |
| MAC | Macrolides: clarithromycin/azithromycin always in combination with ethambutol and a third agent – rifamycin (rifampin/rifabutin) or aminoglycoside (streptomycin/amikacin) | 6–12 months treatment with at least 3 drugs recommended Excisional surgery or debridement often needed |
| M. haemophilum | Amikacin, clarithromycin, ciprofloxacin, rifampin, and rifabutin. Doxycycline and sulfonamides have shown variable susceptibility | All isolates resistant to ethambutol. A minimum of 6 months treatment is required. |
| M. marinum | Susceptible to rifampin, rifabutin, ethambutol, clarithromycin, sulfonamides, and trimethoprim-sulfamethoxazole; intermediately susceptible to streptomycin, doxycycline and minocycline. | Resistant to isoniazid and pyrazinamide. Usually two active agents given for 1–2 months after resolution of symptoms – 3–4 months in total. |
| M. ulcerans | Combination of rifampicin and clarithromycin is the recommended treatment. | Rifampin 10 mg/kg/day with clarithromycin 7.5 mg/kg twice daily for 8 weeks. Telacebec is a new anti-tuberculosis drug with potent activity aganist M. ulcerans. Surgical debridement combined with skin grafting is recommended. |
RGM: Rapid-growing mycobacteria, M. fortuitum: Mycobacterium fortuitum, M. chelonae: Mycobacterium chelonae, M. abscessus: Mycobacterium abscessus, SGM: slow-growing mycobacteria, MAC: Mycobacterium avium complex, M. haemophilum: Mycobacterium haemophilum, M. marinum: Mycobacterium marinum, M. ulcerans: Mycobacterium ulcerans
In general, antibiotic susceptibility should be performed in all RGM species because of unpredictable resistance patterns.[3] Inducible macrolide resistance encoded by erm(41)/erm(39) gene is of particular concern.[2,7]
Newer agents
Delamanid and bedaquiline, two new anti-tuberculosis drugs, have activity against NTM, including MAC, M. abscessus, and M. Ulcerans. Tedizolid is emerging as a more tolerable alternative to linezolid. There are isolated reports of use of omadacycline in the treatment of cutaneous infections due to NTM.[55] Avibactam, a novel beta-lactamase inhibitor can restore susceptibility to beta-lactams in resistant M. abscessus strains.[7]
CONCLUSION
Skin infections caused by NTM are challenging in many ways, from non-specific presentations, difficulty in microbiological diagnosis, lack of well-defined treatment guidelines, and varying patterns of antimicrobial resistance. As a group, the incidence of NTM infections is on the rise; hence, it is important for the clinicians to have an in-depth understanding of the condition.
Acknowledgments
The author is grateful to Dr Deepa M. Nair, Consultant Microbiologist, Medical Trust Hospital, Kochi for guidance and advice on microbiological diagnosis of non-tuberculous mycobacterial infection.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
Dr. Mamatha George is on the editorial Board of the journal.
Financial support and sponsorship
Nil.
References
- Nontuberculous mycobacteria: Skin and soft tissue infections. Dermatol Clin. 2015;33:563-77.
- [CrossRef] [PubMed] [Google Scholar]
- An official ATS/IDSA statement: Diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367-416.
- [CrossRef] [PubMed] [Google Scholar]
- Cutaneous infections due to nontuberculosis Mycobacterium: Recognition and management. Am J Clin Dermatol. 2018;19:867-78.
- [CrossRef] [PubMed] [Google Scholar]
- Atypical mycobacterial cutaneous infections. Dermatol Clin. 2009;27:63-73.
- [CrossRef] [PubMed] [Google Scholar]
- Anonymous mycobacteria in pulmonary disease. Med Clin North Am. 1959;43:273-90.
- [CrossRef] [PubMed] [Google Scholar]
- Whole-genome sequencing to identify transmission of Mycobacterium abscessus between patients with cystic fibrosis: A retrospective cohort study. Lancet. 2013;381:1551-60.
- [CrossRef] [PubMed] [Google Scholar]
- Skin and soft tissue infections due to nontuberculous mycobacteria. Curr Infect Dis Rep. 2018;20:6.
- [CrossRef] [PubMed] [Google Scholar]
- Reduced glutaraldehyde susceptibility in Mycobacterium chelonae associated with altered cell wall polysaccharides. J Antimicrob Chemother. 1999;43:759-65.
- [CrossRef] [PubMed] [Google Scholar]
- Increased incidence of cutaneous nontuberculous mycobacterial infection, 1980 to 2009: A population-based study. Mayo Clin Proc. 2013;88:38-45.
- [CrossRef] [PubMed] [Google Scholar]
- Mendelian susceptibility to mycobacterial disease: Recent discoveries. Hum Genet. 2020;139:993-1000.
- [CrossRef] [PubMed] [Google Scholar]
- Cutaneous nontuberculous mycobacterial infection in Thailand: A 7-year retrospective review. Medicine (Baltimore). 2020;99:e19355.
- [CrossRef] [PubMed] [Google Scholar]
- An unusual acid-fast infection of the knee with subcutaneous, abscess-like lesions of the gluteal region; Report of a case with a study of the organism, Mycobacterium abscessus, n. sp. J Invest Dermatol. 1953;20:133-69.
- [CrossRef] [PubMed] [Google Scholar]
- Mycobacterium abscessus: A cutaneous infection in a patient on renal replacement therapy. Clin Exp Dermatol. 2001;26:415-8.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical patterns of cutaneous nontuberculous mycobacterial infections. Br J Dermatol. 2005;152:727-34.
- [CrossRef] [PubMed] [Google Scholar]
- An outbreak of post-surgical infection due to Mycobacterium abscessus. Pediatr Surg Int. 1998;13:406-10.
- [CrossRef] [PubMed] [Google Scholar]
- Nontuberculous mycobacterial infections after cosmetic surgery-Santo Domingo, Dominican Republic, 2003-2004. MMWR Morb Mortal Wkly Rep. 2004;53:509.
- [Google Scholar]
- Sporotrichoid dermatosis caused by Mycobacterium abscessus from a public bath. J Dermatol. 2000;27:264-8.
- [CrossRef] [PubMed] [Google Scholar]
- Mycobacterium abscessus bacteremia after receipt of intravenous infusate of cytokine-induced killer cell therapy for body beautification and health boosting. Clin Infect Dis. 2013;57:981-91.
- [CrossRef] [PubMed] [Google Scholar]
- Catheter-related Mycobacterium abscessus bacteremia manifested with skin nodules, pneumonia, and mediastinal lymphadenopathy. Kaohsiung J Med Sci. 2013;29:50-4.
- [CrossRef] [PubMed] [Google Scholar]
- Mycobacterium chelonae infection complicating traumatic and surgical wounds: A case series. Indian J Dermatol Venereol Leprol. 2018;84:45-8.
- [CrossRef] [PubMed] [Google Scholar]
- Hospital outbreak of atypical mycobacterial infection of port sites after laparoscopic surgery. J Hosp Infect. 2006;64:344-7.
- [CrossRef] [PubMed] [Google Scholar]
- Mycobacterium chelonae: Nonhealing leg ulcers treated successfully with an oral antibiotic. J Am Board Fam Pract. 2001;14:457-61.
- [Google Scholar]
- Mycobacterium chelonae infection associated with adalimumab therapy. Scand J Rheumatol. 2008;37:159-60.
- [CrossRef] [PubMed] [Google Scholar]
- Mycobacteria other than Mycobacterium tuberculosis: Review of microbiologic and clinical aspects. J Infect Dis. 1987;9:275-94.
- [CrossRef] [PubMed] [Google Scholar]
- "Mycobacterium fortuitum" a new pathogenic acid fast bacillus in humans. Acta Med Rio de Janeiro. 1938;1:297-301.
- [Google Scholar]
- Atypical Mycobacterium furunculosis occurring after pedicures. J Am Acad Dermatol. 2006;54:520-4.
- [CrossRef] [PubMed] [Google Scholar]
- An outbreak of mycobacterial furunculosis associated with contaminated whirlpool footbaths at a nail salon. N Engl J Med. 2002;346:1366-71.
- [CrossRef] [PubMed] [Google Scholar]
- Mycobacterium fortuitum infection of a hemodialysis catheter in a pediatric patient. Hemodial Int. 2019;23:E93-6.
- [CrossRef] [PubMed] [Google Scholar]
- Spontaneous tuberculosis in salt water fish. J Infect Dis. 1926;39:315-20.
- [CrossRef] [Google Scholar]
- Sixty-three cases of Mycobacterium marinum infection: Clinical features, treatment, and antibiotic susceptibility of causative isolates. Arch Intern Med. 2002;162:1746-52.
- [CrossRef] [PubMed] [Google Scholar]
- Mycobacterium marinum causing tenosynovitis. 'Fish tank finger' Acta Orthop Belg. 2004;70:279-82.
- [Google Scholar]
- Sequelae of World War II: An outbreak of chronic cutaneous nontuberculous mycobacterial infection among Satowanese Islanders. Clin Infect Dis. 2009;48:1541-6.
- [CrossRef] [PubMed] [Google Scholar]
- Cutaneous Mycobacterium marinum infection ("swimming pool granuloma") Clin Dermatol. 1987;5:68-75.
- [CrossRef] [PubMed] [Google Scholar]
- Mycobacterium marinum tenosynovitis in a patient on etanercept therapy for rheumatoid arthritis. J Clin Rheumatol. 2002;8:265-8.
- [CrossRef] [PubMed] [Google Scholar]
- Severe sporotrichoid fish tank granuloma following infliximab therapy. Am J Clin Dermatol. 2007;8:385-8.
- [CrossRef] [PubMed] [Google Scholar]
- Mycobacterium other than tuberculosis (MOTT) infection: An emerging disease in infliximab-treated patients. J Infect. 2007;55:484-7.
- [CrossRef] [PubMed] [Google Scholar]
- A case of opportunistic skin infection with Mycobacterium marinum during adalimumab treatment in a patient with Crohn's disease. J Crohns Colitis. 2013;7:e15-8.
- [CrossRef] [PubMed] [Google Scholar]
- Buruli Ulcer: Review of a neglected skin mycobacterial disease. J Clin Microbiol. 2018;56:e01507-17.
- [CrossRef] [PubMed] [Google Scholar]
- Buruli Ulcer: A review of the current knowledge. Curr Trop Med Rep. 2018;5:247-56.
- [CrossRef] [PubMed] [Google Scholar]
- Primary cutaneous infection by Mycobacterium avium: A case report and literature review. Cutis. 2012;89:175-9.
- [Google Scholar]
- Cutaneous mycobacterial infections. Clin Microbiol Rev. 2019;32:e00069-18.
- [CrossRef] [PubMed] [Google Scholar]
- Skin and soft tissue infection by Mycobacterium intracellulare in an immunocompetent patient. IDCases. 2020;19:e00720.
- [CrossRef] [PubMed] [Google Scholar]
- Papulonecrotic tuberculid secondary to disseminated Mycobacterium avium complex. Int J Dermatol. 1994;33:109-12.
- [CrossRef] [PubMed] [Google Scholar]
- Mycobacterium chimaera infections: An update. J Infect Chemother. 2020;26:199-205.
- [CrossRef] [PubMed] [Google Scholar]
- Disseminated Mycobacterium chimaera presenting as vertebral osteomyelitis. Case Rep Infect Dis. 2017;2017:9893743.
- [CrossRef] [PubMed] [Google Scholar]
- Ulcerating nodules on the face due to Mycobacterium chimaera in a patient with diabetes. Clin Exp Dermatol. 2022;47:587-9.
- [CrossRef] [PubMed] [Google Scholar]
- Disseminated Mycobacterium haemophilum infection. Lancet Infect Dis. 2011;11:571-8.
- [CrossRef] [PubMed] [Google Scholar]
- rpoB gene sequencing for identification of rapidly growing mycobacteria. Arch Pediatr Infect Dis. 2017;5:e40001.
- [CrossRef] [Google Scholar]
- Cutaneous infections due to nontuberculous mycobacteria: Histopathological review of 28 cases. Comparative study between lesions observed in immunosuppressed patients and normal hosts. J Cutan Pathol. 2000;27:124-9.
- [CrossRef] [PubMed] [Google Scholar]
- Out of Africa-observations of histopathology of Mycobacterium ulcerans. Clin Pathol. 1996;43:5-9.
- [CrossRef] [PubMed] [Google Scholar]
- The histopathologic spectrum in Mycobacterium marinum infection. Arch Pathol Lab Med. 1985;109:1109-13.
- [Google Scholar]
- Mycobacterial skin infection. Curr Opin Infect Dis. 2022;35:79-87.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment of Mycobacterium ulcerans Disease (Buruli Ulcer): Guidance for Health Workers. 2012. Available from: https://www.apps.who.int/iris/handle/10665/77771 [Last accessed on 2023 Jan 20]
- [Google Scholar]
- Treatment and prevention of Mycobacterium ulcerans infection (Buruli ulcer) in Australia: Guideline update. Med J Aust. 2014;200:267-70.
- [CrossRef] [PubMed] [Google Scholar]






