Translate this page into:
Photosensitive presentation of aggressive mycosis fungoides mimicking systemic lupus erythematosus
*Corresponding author: Pradeep S. Nair, Department of Dermatology and Venereology, Government Medical College, Trivandrum, Kerala, India. dvmchtvm@yahoo.co.in
-
Received: ,
Accepted: ,
How to cite this article: Nair PS, Prathap A, Sudhesan A. Photosensitive presentation of aggressive mycosis fungoides mimicking systemic lupus erythematosus. J Skin Sex Transm Dis. 2024;6:176-9. doi: 10.25259/JSSTD_51_2023
Abstract
A 62-year-old female with a history of carcinoma cervix presented with fever, oral erosions, joint pains, and photosensitivity of 6 months duration. On examination, the patient had multiple discrete erythematous macules, papules, and plaques on the face and forearms in a photo distribution. The patient was investigated on the lines of systemic lupus erythematosus, but the antinuclear antibody profile was negative. Slit skin smear from skin lesions showed malignant lymphocytes. Peripheral smear and bone marrow smear showed malignant lymphocytes. Skin biopsy showed the entire dermis packed with malignant lymphocytes. Immunocytochemistry showed malignant lymphocytes which are CD3+, CD4+, CD30 negative, CD20 and CD117 negative, diagnostic of mycosis fungoides (MF). The patient expired before the treatment could be started. We are reporting an aggressive form of MF presenting with the clinical features suggestive of systemic lupus erythematosus.
Keywords
Mycosis fungoides
Systemic lupus erythematosus
Photosensitive
INTRODUCTION
Mycosis fungoides (MF), a primary cutaneous T-cell lymphoma, is a malignant T-cell proliferative disorder of the T-memory cells. MF has a chronic indolent course and, during its evolution through the patch, plaque, tumor, and erythrodermic phase, it can mimic many dermatoses and, therefore, is “a great imitator.”[1] Multiple skin biopsies may be essential for initial diagnosis in the early phases, and diagnosis may be missed if a strong index of suspicion is not entertained. However, occasionally it can present with a short, aggressive course and confound diagnosis.[2] Here, we are reporting a case of aggressive MF clinically presenting with features of systemic lupus erythematosus (SLE), another “great imitator.”
CASE REPORT
A 62-year-old female presented with complains of reddish raised, itchy skin lesions of the face, front of chest, and forearms of 6 months duration. The lesions first started on the face, and later, the front of the chest and forearms. The itching and erythema increased on exposure to sunlight. Two months ago, the patient also noticed painless erosions on the hard palate, which was painless. The patient also gave a history of pain in knee and knuckle joints. She gave a history of carcinoma cervix one year ago, which was treated with radiation and chemotherapy. Recently, the patient complained of recurrent fever, weight loss, fatigue, and muscle weakness. On examination, the patient was ill-looking, and there were well-defined erythematous macules, papules, and plaques distributed on the face, V area of the chest [Figure 1], and forearms [Figure 2]. There were no skin lesions elsewhere other than generalized xerosis. The oral cavity showed a large ill-defined erosion on the hard palate. Musculoskeletal examination showed arthralgia and restricted movement of knee and ankle joints. There was no muscle tenderness. There was no generalized lymphadenopathy or hepatosplenomegaly.
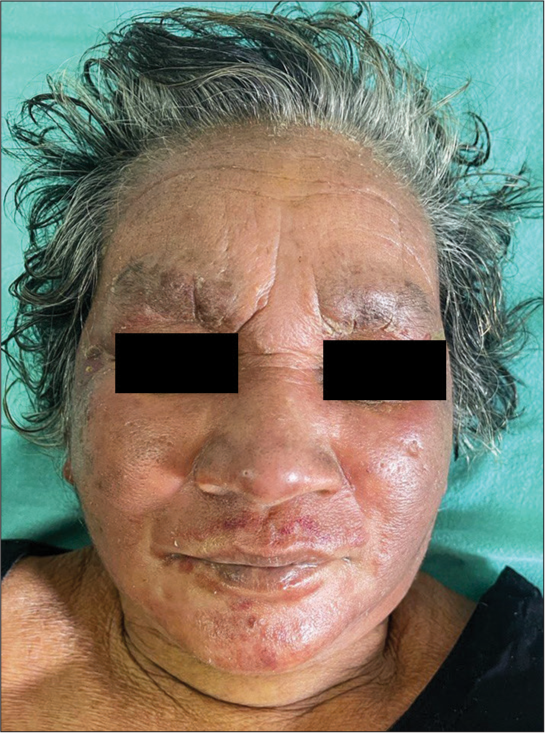
- Erythema, edema of the forehead, cheeks, and nose.
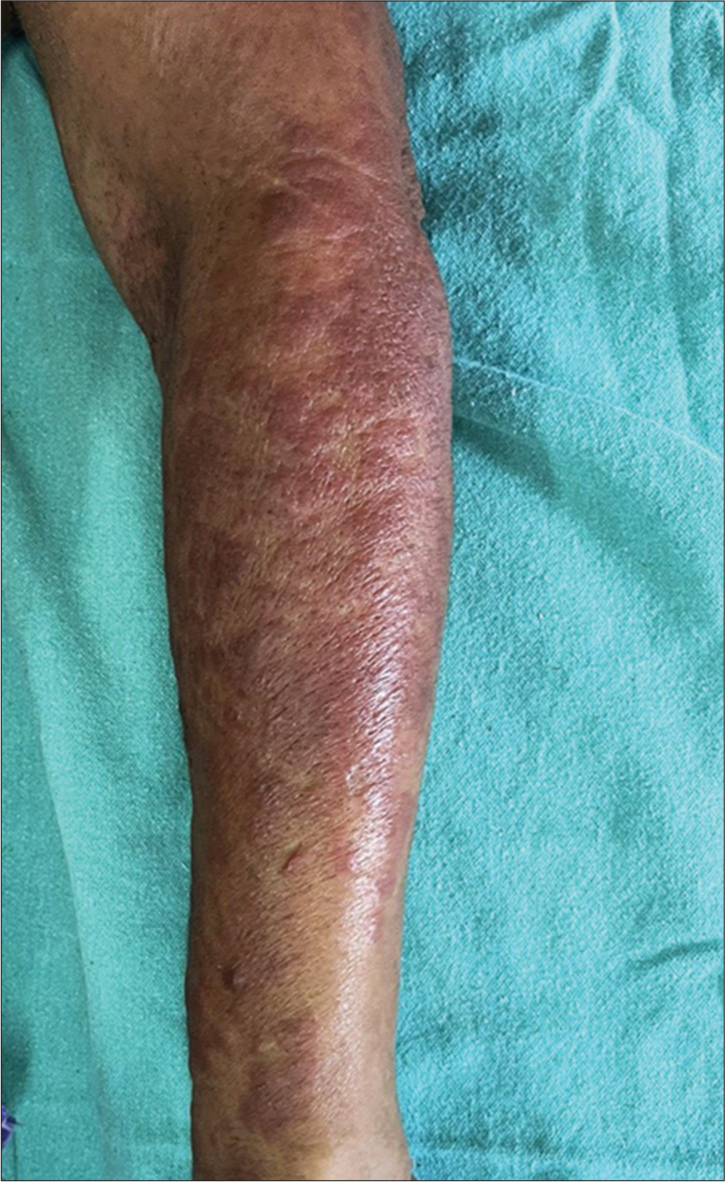
- Erythematous papules and plaques on the forearm.
A provisional diagnosis of SLE, and due to generalized fatigue and weakness, SLE-dermatomyositis overlap were considered. Hemogram showed anemia (8 gm%) and an erythrocyte sedimentation rate of 70 mm/hr. Liver function tests were normal, while renal function tests showed elevated serum urea (52 mg%) and creatinine (3.2 mg%). Serum calcium was 8.1 mg%, sodium 122 mg%, and potassium 3.9 mg%. Serum creatine phosphokinase was 112 U/L, and lactate dehydrogenase was 183 U/L. Viral markers were negative. Chest X-ray and ultrasound of the abdomen were normal. The antinuclear antibody profile was negative. A slit skin smear (SSS) from the erythematous plaque on the forearm stained by Leishman stain showed atypical large mononuclear cells with hyperchromatic nuclei [Figure 3a]. Peripheral smear showed neutrophilia with atypical large mononuclear cells with hyperchromatic nuclei almost filling the cell [Figure 3b]. A skin biopsy showed massive infiltration of the upper and mid-dermis with mononuclear atypical cells [Figure 4]. There were no histological features to suggest SLE. Immunohistochemistry (IHC) staining of the skin biopsy specimen demonstrated CD3 positive, CD4 positive [Figure 5a and b], and CD30 negative, lymphocytes while CD20 and CD117 were negative. Bone marrow was CD3+ lymphocytes. All these findings were diagnostic of MF/primary cutaneous T-cell lymphoma. We made a final diagnosis of aggressive MF presenting with clinical features of SLE.
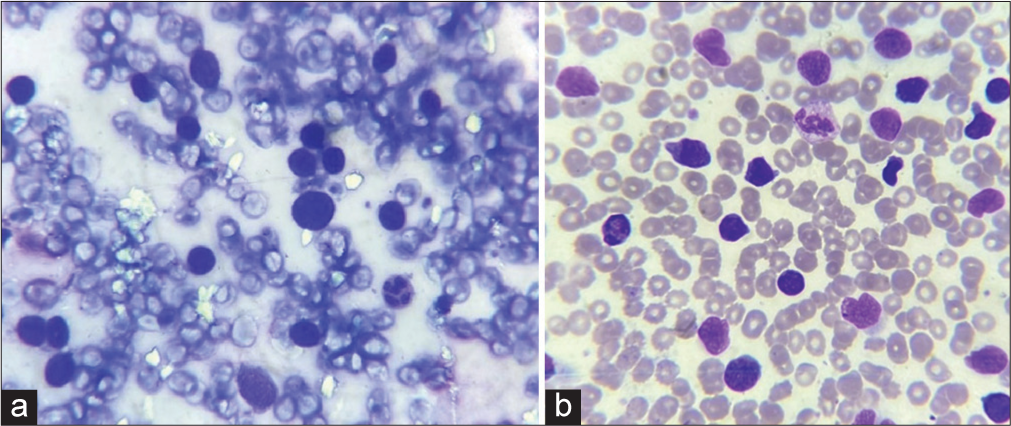
- (a): Slit skin smear showing atypical cells with hyperchromatic nuclei, (Leishman stain, ×1000); (b): peripheral smear showing malignant lymphocytes, (Leishman stain, ×1000).
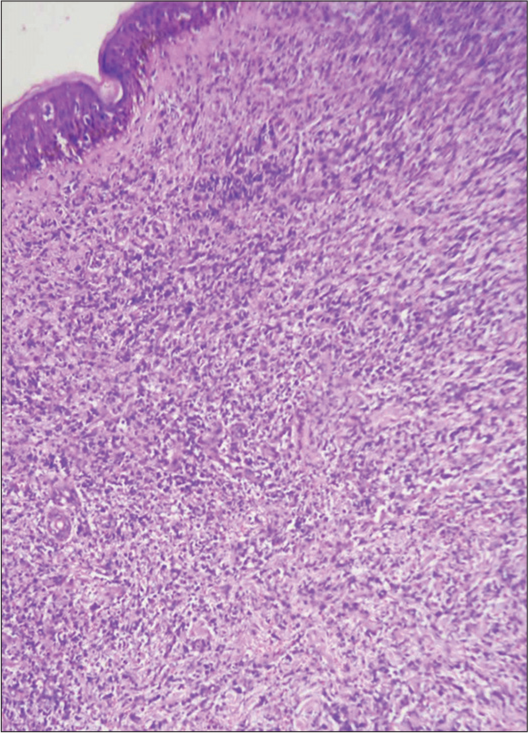
- Skin biopsy showing the dermis packed with atypical mononuclear cells, hematoxylin & eosin, ×200.
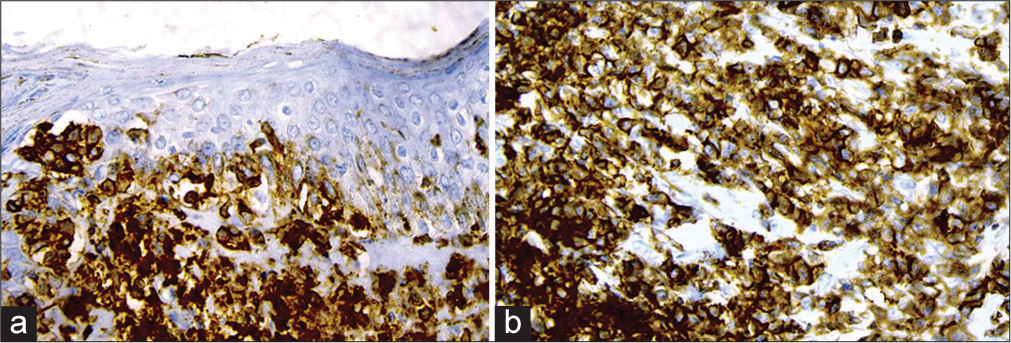
- (a): Immunohistochemistry showing CD3 positive; (b): CD4 positive cells, ×400.
DISCUSSION
Our patient presented with a history of fever, general malaise, photosensitivity, oral erosions, and joint pains. The patient had well-defined erythematous plaques on the photo-exposed sites, the other sites being spared. Hence, we considered SLE, but investigations did not support a diagnosis of SLE. Further, investigations due to worsening of the clinical condition of the patient clinched the diagnosis of MF confirmed by IHC. We made a diagnosis of aggressive MF as the total duration of the disease process was only six months, and the patient expired within 3 weeks after diagnosis.
Initial clinical presentation with photosensitivity, oral erosions, and arthralgia mimicked SLE. The usual course of MF is a chronic, indolent nature extending over many years.[3] An aggressive type of MF is seen in CD30-positive cases; however, in our case, this was negative.[4] Blood examination showed atypical cells in the peripheral smear, but a diagnosis of Sezary syndrome (SS) could not be diagnosed made as there was no generalized lymphadenopathy or exfoliative dermatitis. The skin lesions were predominantly papules and plaques when we should expect tumor in such aggressive MF. We could not assess visceral involvement as the patient expired before CT-scan/MRI imaging could be done. We do not have a clear explanation of how a CD30 negative, non-tumor stage MF or absence of SS could account for a highly aggressive type of MF. It is possible that the previous history of carcinoma cervix treated with chemotherapy and radiation may have contributed to severe immunosuppression in our patient, leading to an aggressive and fatal outcome of MF. Erythematous skin lesions with peripheral blood involvement and aggressive course are also features of adult T-cell leukemia/lymphoma (ATLL). The most important clinical feature of ATLL is generalized lymphadenopathy, which was not seen in our case. In ATLL, peripheral smear shows “flower cells,” which was not seen in our case. IHC in ATLL also shows CD4+ cells like MF, but, in our case, there were both CD3+ and CD4+, and the bone marrow was also positive for CD3, all suggestive of with MF. MF has protean manifestations and can mimic eczemas, papulosquamous dermatosis, infections, and SLE, as in the present case.[5] Epidermotropism and Pautrier microabscess are the classical histopathological features in early MF but may not be seen in the late stages and aggressive cases as the present case.[6] There are isolated reports of photosensitive and SLE-like presentation of MF in literature, but all these reports had a favorable outcome, unlike our case.[7,8] MF presenting as actinic reticuloid has also been reported in the literature, but these cases had a chronic indolent course.[9] Photosensitive MF may cause management problems as psoralen ultraviolet A and narrow-band ultraviolet-B are established therapeutic options.[10] Another important highlight of our case was that we could demonstrate malignant cells by SSS, which is a simple technique and can be easily done before a skin biopsy. We are reporting a rare and unique case of a “great imitator” – MF presenting as another “great imitator” – SLE with an aggressive and fatal outcome.
CONCLUSION
MF is a cutaneous T-cell lymphoma which is a great imitator and we are reporting a case of MF presenting with features suggestive of SLE.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
Dr. Pradeep S. Nair is on the editorial board of the Journal.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Epidemiology and prognostic factors for mycosis fungoides and Sézary syndrome in a multi-ethnic Asian cohort: A 12-year review. J Eur Acad Dermatol Venereol. 2019;33:1513-21.
- [CrossRef] [PubMed] [Google Scholar]
- CD30+ large cell transformation of mycosis fungoides during pregnancy. Indian J Dermatol. 2013;58:160.
- [CrossRef] [PubMed] [Google Scholar]
- Mycosis fungoides: A clinicopathological study of 60 cases from a tertiary care center. Indian J Dermatol. 2020;65:123-9.
- [CrossRef] [PubMed] [Google Scholar]
- Mycosis fungoides: A retrospective study of 44 Swedish cases. Acta Derm Venereol. 2016;96:669-73.
- [CrossRef] [PubMed] [Google Scholar]
- Clinicopathological spectrum of cutaneous lymphomas and distinction of early mycosis fungoides from its mimics-a retro-prospective descriptive study from Southern India. Indian Dermatol Online J. 2022;13:737-46.
- [CrossRef] [PubMed] [Google Scholar]
- A clinicopathological analysis of primary cutaneous lymphomas: A 6-year observational study at a tertiary care center of South India. Indian J Dermatol. 2016;61:608-17.
- [CrossRef] [PubMed] [Google Scholar]
- Photo-sensitive mycosis fungoides: A new variant? Eur J Dermatol. 2017;27:181-2.
- [CrossRef] [PubMed] [Google Scholar]
- Mycosis fungoides with photosensitivity mimicking chronic actinic dermatitis. Indian Dermatol Online J. 2021;12:337-9.
- [CrossRef] [PubMed] [Google Scholar]
- Prognostic factors, prognostic indices and staging in mycosis fungoides and Sézary syndrome: Where are we now? Br J Dermatol. 2014;170:1226-36.
- [CrossRef] [PubMed] [Google Scholar]
- Localized mycosis fungoides treated with laser-assisted photodynamic therapy: A case series. Clin Exp Dermatol. 2019;44:930-2.
- [CrossRef] [PubMed] [Google Scholar]






