A hospital-based single-arm study to assess the efficacy of intralesional bleomycin injection in patients of resistant periungual and palmoplantar warts
*Corresponding author: Shivani Ranjan, Assistant Professor, Department of Dermatology, Government Medical College, Jammu, Jammu and Kashmir, India. drshivaniranjan@rocketmail.com
-
Received: ,
Accepted: ,
How to cite this article: Ranjan S, Sharma P, Dogra D. A hospital-based single-arm study to assess the efficacy of intralesional bleomycin injection in patients of resistant periungual and palmoplantar warts. J Skin Sex Transm Dis. 2024;6:144-9. doi: 10.25259/JSSTD_39_2024
Abstract
Objectives:
The objective of the study is to assess the efficacy of intralesional (I/L) bleomycin therapy for the treatment of resistant periungual and palmoplantar warts.
Materials and Methods:
A total of 51 patients of age ≥13 years with periungual and palmoplantar warts for ≥3-month duration and non-responsive to at least two conventional treatment modalities were included in this study and treated with I/L bleomycin (1.5 mg/mL solution) every 2 weeks for maximum up to two injections. The patients were followed up every 2 weeks up to 3 months. If warts did not regress in size after 3 months of starting treatment, it was considered as failure.
Results:
A total of 51 patients were included in the study who presented with 146 warts. 38 (74.5%) patients were treated with a single dose of I/L bleomycin, and 13 (25.5%) patients received two doses at an interval of 2 weeks. Complete clearance (CC) was seen in 37 (72.5%) at 2 weeks and near CC (NCC) in 13 (25.6%) of patients at 4 weeks. Recurrence was observed in one patient (1.9%) after achieving NCC initially. Mild-to-moderate pain was the most common side effect (98%) reported, which lasted up to 2-3 days in 20% of the patients, with a peak at the time of injection. Perilesional hyperpigmentation was seen in 41 (80.3%) patients that gradually faded during the follow-up of 12 weeks. Eschar formation and necrosis of lesions were seen in 7 (13.7%) and 3 (5.8%) patients, respectively. A single patient experienced Raynaud’s phenomenon who finally was lost to follow-up.
Limitations:
Limited number of patients, lack of control group, and short follow up period were the main limitations of the present study.
Conclusion:
Intralesional bleomycin is a highly effective and safe treatment option for periungual and palmoplantar warts, which are usually resistant to conventional treatment modalities..
Keywords
Bleomycin
Intralesional injection
Palmoplantar
Periungual warts
INTRODUCTION
Warts are one of the most common viral infections affecting the skin caused by the human papillomavirus. A huge armamentarium of treatments is available, including salicylic acid, lactic acid, imiquimod, electrocautery, chemical cauterization, surgical excision, curettage, cryotherapy, podophyllotoxin, 5-fluorouracil (topical or intralesional [I/L]), immunotherapy, photodynamic therapy, and pulsed dye laser. However, no single therapeutic modality has been fully successful so far. Furthermore, recurrences and scarring are possible with conventional modalities. Treatment of resistant palmoplantar warts at difficult sites is even more exigent. Many studies have confirmed the efficacy and safety of I/L bleomycin for the management of cutaneous warts.[1-5] However, at present, there is still paucity of evidence of I/L bleomycin for treating resistant palmoplantar and difficult site periungual/subungual warts. Therefore, we conducted this study for the assessment of efficacy and safety of I/L bleomycin for the treatment of palmoplantar and periungual/subungual warts which are customarily difficult to treat with other conventional therapeutic modalities.
Bleomycin was originally isolated by Umezawa and coworkers from the fungus Streptomyces verticillus in 1962.[6] This glycopeptide has antibacterial, antiviral, and cytotoxic effects. The mechanism of action of bleomycin involves DNA and RNA disintegration and free radical production, causing inhibition of the cell cycle at the G2 phase, ultimately leading to the death of the tumor cells. It also causes endothelial damage.[7]
MATERIALS AND METHODS
This is a prospective study done over a period of 2 years from June 2020 to June 2022. The study was conducted after obtaining ethical committee clearance as well as informed and written consent from all patients. Fifty-one patients (13–70 years of age) with multiple palmoplantar and periungual warts of duration 3 months or more and non-responsive to at least two conventional treatment modalities (resistant warts) were included in the present study. Exclusion criteria included pregnant and lactating women and patients with a history of peripheral vascular disease, pulmonary fibrosis, or any other systemic illness.
The site, number, and size of warts selected for treatment were recorded at baseline and during follow-up. Patients were followed up every 2 weeks for a period of 3 months. Serial photographic assessment and clinical assessment were made during each visit to look for the efficacy and safety profile of the treatment as well as for recurrence. Investigations, including complete blood count, liver function tests, renal function tests, blood sugar fasting, and human immunodeficiency virus testing, were done at baseline and at the end of the follow-up period. At each visit, any possible systemic or cutaneous side effects (including pain during and after treatment, pigment changes, Raynaud’s phenomenon, scarring, nail changes, itching, and skin rash) and their severity were noted. The pain was assessed by oral questionnaires such as no pain (0), mild pain (1–3) (noticeable but does not interfere with daily activities), moderate pain (4–6) (interfere with daily activities but controllable), and severe pain (7–10) (disabling, patient is unable to perform daily activities) on 0–10 Numerical Rating Scale. Patients were also examined for any systemic side effects. Clinical improvement in lesions was categorized as complete clearance (CC) (100%), near CC (NCC) (75–99%), significant clearance (50–74%), moderate clearance (25–49%), mild clearance (1–25%), and no clearance (0%). “Recurrence” was defined as the reappearance of warts during the study period. If the warts did not regress in size at 3 months of starting treatment, it was considered as a “failure” of treatment. Data obtained from all patients was tabulated and summarized.
Statistical analysis was performed data using Microsoft Word Excel software through various numerical and graphical techniques.
Commercially, bleomycin is available as vials containing 15 mg powder. Stock solution is prepared by diluting it with 5 mL of distilled water, which can be stored in the refrigerator for 2 months at 4–8°C. One part of this solution was mixed with 1 part of 2% lidocaine so that the final concentration became 1.5 mg/mL = 1.5 U/mL. Lidocaine was added as a diluent to reduce pain during and immediately after the procedure. Each wart was cleansed with isopropyl alcohol swab. Slight superficial paring was done to remove the callus tissue in the surrounding lesion. Then, the bleomycin injection was given at the base of the warts intralesionally till blanching of the lesion occurred.
The amount of the injection given depends on the size of warts. For warts up to 5 mm, 10 mm, and more than 10 mm, 0.2 mL, 0.5 mL, and 1 mL of I/L bleomycin was injected, respectively. To avoid any systemic side effects, 1 mL was set as the limit for the total volume to be injected in one treatment sitting. If a black, ecchymosed eschar was present at 2 weeks of bleomycin injection, it was pared, and residual warts, if seen, were injected a 2nd time. Clinical improvement in lesions was analyzed by photographic assessment.
RESULTS
Table 1 shows the baseline characteristics of patients. Fifty-one (51) patients were enrolled in the study. There were 30 (58.8%) males and 21 (41.2%) females. Patients’ ages ranged from 13 to 70 years, with mean age being recorded as 31.31 years (standard deviation [SD] = 11.64). The duration of warts ranged from a minimum of 3 months to a maximum of 36 months, with a mean duration of 12.3 months (SD = 7.05). One hundred and forty-six (146) warts present in 51 patients were subjected to I/L therapy in the present study. Warts were distributed over various sites, including plantar (50.9%), palmar (37.2%), periungual (9.8%), subungual area (5.9%), and at multiple sites (1.9%). Table 2 shows number of sessions, clinical outcome [Figure 1], and adverse effects of I/L bleomycin therapy. A total of 38 (74.5%) patients were treated with a single dose of I/L bleomycin in a dosage of 1.5 mg/mL, and 13 (25.5%) patients received two doses at an interval of 2 weeks. CC was seen in 37 (72.5%) at 2 weeks [Figures 2 and 3] and NCC [Figure 4] in 13 (25.6%) of patients at 4 weeks. Recurrence was observed in one patient (1.9%) at a 12-week follow-up after showing initial NCC at 4 weeks. Mild-to-moderate pain was the most common side effect (98%), which lasted up to 2–3 days in 20% of the patients, with a peak at the time of injection, for which tablet diclofenac was advised. Warts regressed without any scarring but with slight perilesional hyperpigmentation (PLH) in 41 (80.3%) patients [Figure 2] that gradually faded during the follow-up of 3 months. Eschar formation (hemorrhagic crust) [Figure 5] and necrosis of lesions were seen in 7 (13.7%) and 3 (5.8%) patients, respectively. A single patient experienced Raynaud’s phenomenon after 1 day of procedure limited to the injected fingers, for which he was advised to take a tablet of nifedipine 5 mg once daily, and finally, the patient was lost to follow-up. None of the patients following I/L injection around the nails observed any effect on nail growth or nail anatomy.
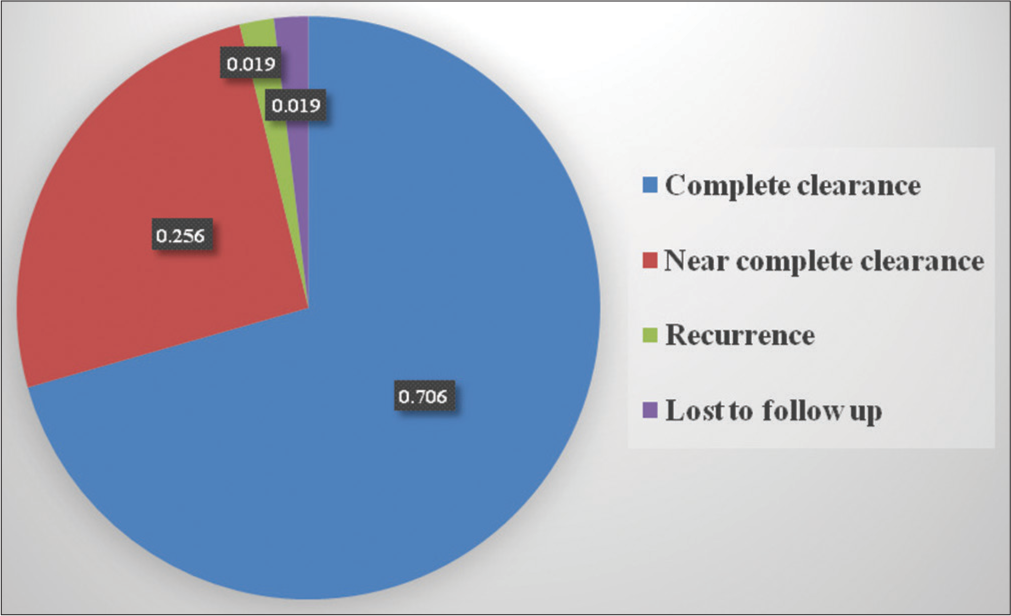
- Pie chart representation of response following intralesional bleomycin.
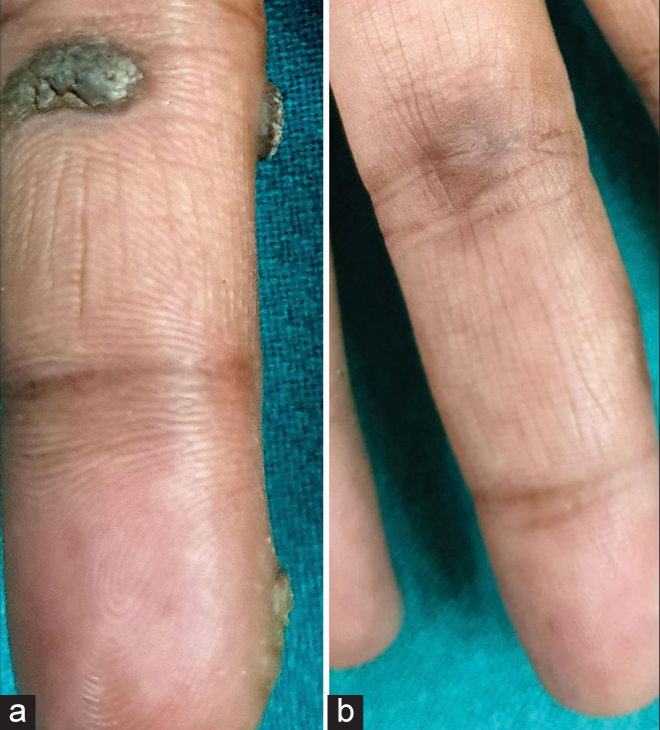
- (a) Palmar wart at baseline. (b) At 2 weeks after single sitting of intralesional bleomycin showing complete clearance with residual hyperpigmentation.
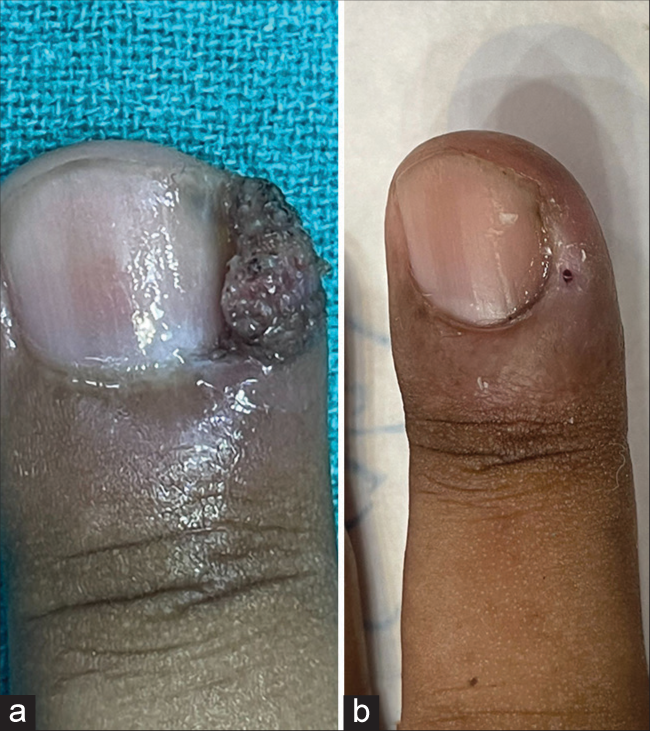
- (a) Periungual wart at baseline. (b) Complete clearance at 2 weeks.
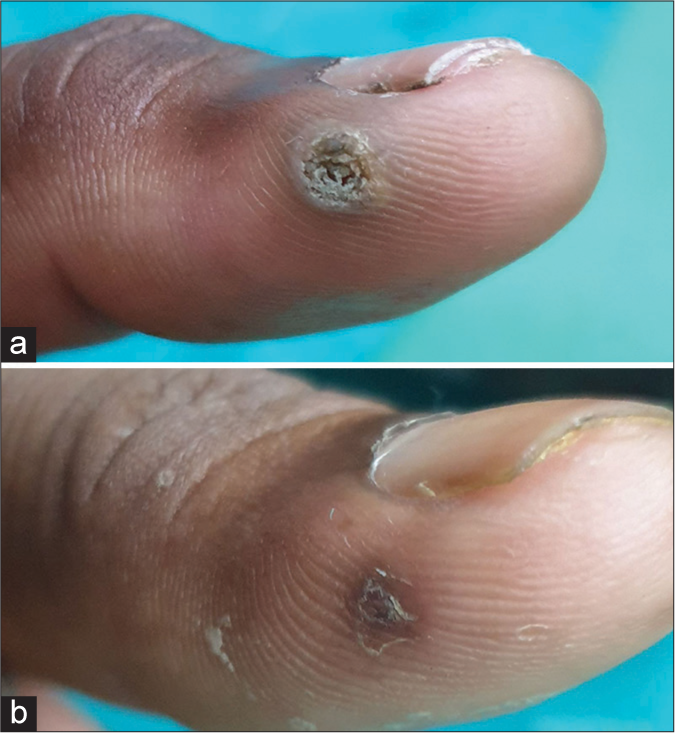
- (a) Palmar wart at baseline. (b) Near complete clearance after two sittings of intralesional bleomycin.
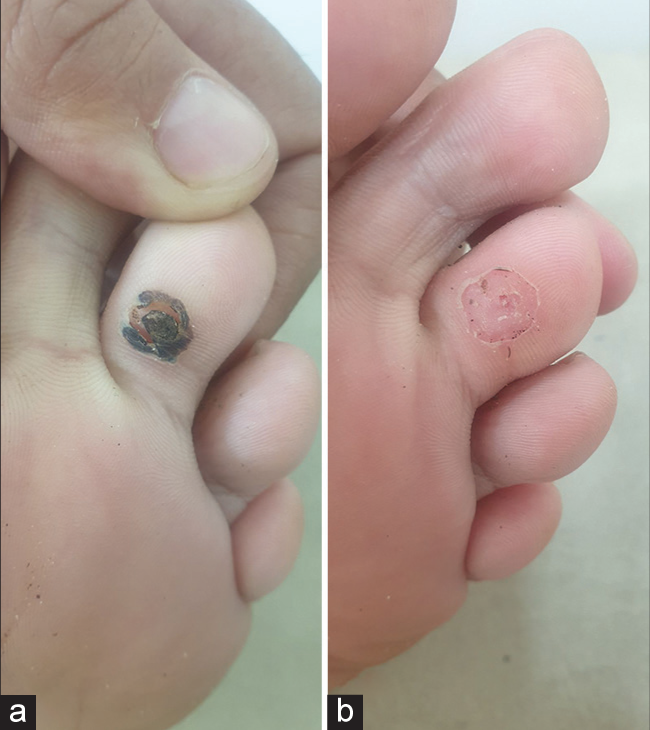
- (a) Plantar wart showing Eschar formation post intralesional bleomycin. (b) Complete clearance of wart after paring.
| Baseline characteristics | No. of patients (n=51) (%) |
|---|---|
| Gender | |
| Male | 30 (58.8) |
| Female | 21 (41.2) |
| Age (years) | |
| <20 | 6 (11.8) |
| 20–40 | 37 (72.5) |
| >40 | 8 (15.7) |
| Duration of warts (months) | |
| 1–6 | 10 (19.6) |
| 7–12 | 28 (54.9) |
| >12 | 13 (25.5) |
| No of warts | |
| <5 | 43 (84.4) |
| 5–10 | 7 (13.7) |
| >11 | 1 (1.9) |
| Site of warts | |
| Plantar | 26 (50.9) |
| Palmar | 19 (37.2) |
| Periungual | 5 (9.8) |
| Subungual | 3 (5.9) |
| Multiple sites | 1 (1.9) |
| Clinical data | No. of patients (n=51) (%) |
|---|---|
| Number of sessions of bleomycin | |
| 1 | 38 (74.5) |
| 2 | 13 (25.5) |
| Types of response after bleomycin injection | |
| Complete clearance | 37 (72.5) |
| Near complete clearance | 13 (25.6) |
| Lost to follow-up | 1 (1.9) |
| Types of adverse effects observed | |
| Pain | 50 (98) |
| PLH | 41 (80.3) |
| Eschar | 7 (13.7) |
| Necrosis | 3 (5.8) |
| RP | 1 (1.9) |
RP: Raynaud’s phenomenon, PLH: Perilesional hyperpigmentation
DISCUSSION
Warts can have a strong negative impact on patients’ quality of life.[8] They can cause pain and cosmetic disfigurement depending on their site and are socially unacceptable when present on apparent sites.
I/L bleomycin has shown excellent results for the treatment of palmoplantar and periungual warts in earlier studies, but still, it is an off-label treatment option. Furthermore, a very low dose of bleomycin used in I/L therapy has not been associated with systemic side effects.[4,9] Hence, we wished to determine the efficacy of bleomycin in the treatment of warts at periungual and palmoplantar sites, which are otherwise difficult to treat with conventional modalities such as salicylic acid in petroleum jelly, salicylic acid-lactic acid in collodion, trichloroacetic acid, electric cautery, cryotherapy by liquid nitrogen or carbon dioxide laser, and topical or oral retinoids.
I/L bleomycin has been used in various concentrations (from 1 to 1.5 mg/mL) and techniques to treat warts. In the present study, we observed response to treatment in 96% of patients with CC in 37 (72.5%) and NCC in 13 (25.6%) of patients with I/L bleomycin in a dosage of 1.5 mg/mL and recurrence rate (RR) of 1.9% in 3 months. Whereas in a study conducted by Salk and Douglas using 1.5 mg/mL of I/L bleomycin, a higher cure rate of 87% with one or two injections was observed and 19.35% RR in 6 months.[10] Kruter et al.[11] used 3 mg/mL of bleomycin and observed a comparable cure rate of 74%. Most of the previous studies have used I/L bleomycin in a concentration of 1 mg/ml with comparable complete cure rates ranging from 69% to 96%.[12-17] In a study by Aziz-Jalali et al.,[14] a higher RR of 23% was observed in difficult site recalcitrant warts. Soni et al.[15] achieved 96.47% CC in difficult site warts in patients who did not receive any prior treatment. Singh Mehta et al.[16] observed CC in 84% of patients and partial response in 10% of patients. In a retrospective study by Marahatta et al.,[17] 89.47% of patients achieved CC, and 10.52% achieved NCC with a higher RR of 15.78% seen in 3-month follow-up. Similarly, in a study by Dhar et al.[2] comparing bleomycin (CC - 94.9%) with cryotherapy (CC - 76.5%), 13% RR was observed during the 8-week follow-up period. Lesser CC rate observed in our study might be because we chose a difficult site (periungual/subungual) and resistant warts. However, the present study was associated with only 1.9% RR which was significantly low as compared to most other studies.[2,10,11,17] A study by Barkat et al.[13] has reported 69.3% clearance with dermoscopic evaluation (true clearance), whereas 88.5% clearance when evaluated only clinically.
Mild-to-moderate pain was the most common side effect (98%) reported in the present study, which lasted up to 2–3 days in 20% of the patients, with a peak at the time of injection. Warts regressed without any scarring but with transient PLH in 80.3% of patients. These findings are comparable to other reports.[10-12,17] In a study by Singal and Grover,[18] 62% of patients observed PLH, which gradually faded over 6–8 weeks, and pain was observed in 81.2% of patients. Reversible eschar formation (hemorrhagic crust) and necrosis of lesions were seen in 13.7% and 5.8% of patients, respectively, which subsided with hyperpigmentation. One previous study with I/L bleomycin for ungual warts detected reversible necrosis in 1.2% of the patients.[18] This occurs due to the reduction in blood flow, which causes necrosis and later on disappearance of the lesion. I/L bleomycin should be given with caution in periungual and subungual warts to avoid any damage to the matrix, as it may result in nail deformities. Nail deformity was not observed in the present study. Raynaud’s phenomenon may occur in few days after the procedure or as a delayed side effect.[19,20] In the previous case reports, most cases were young females with warts on their fingers. Raynaud’s phenomenon appeared after the first injection limited to the treated fingers and persisted during follow-ups. However, in the present study, a single young male patient experienced Raynaud’s phenomenon limited to the injected fingers 1 day after the first dose and was advised tablet nifedipine, but later on, the patient was lost to follow-up. The pathogenetic mechanism of this effect is not known.
There are a few limitations of our study. Due to the COVID-19 outbreak during the study period, we could enroll a limited number of patients in the present study. There was a lack of control group. A short follow-up period of 3 months is another constraint of the study. Henceforth, more randomized and double-blind studies of adequate sample size and longer follow-ups are required to further establish the safety and efficacy of I/L bleomycin in the treatment of warts, especially palmoplantar and resistant warts.
CONCLUSION
I/L bleomycin therapy is significantly effective and safe and has better patient acceptance in treating resistant palmoplantar and periungual warts. This treatment does not require any special equipment or setup. It has a low RR. The cost of therapy and the downtime are less as compared to other conventional treatment modalities.
Acknowledgment
Dr. Dev Raj, Sr. Lecturer, Department of Community Medicine for contributing to the statistics of the study.
Ethical approval
The Institutional Review Board has waived the ethical approval for this study, number - IEC/GMC/cat C/2021/466 Reg No. C-117.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript, and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Efficacy of topical treatments for cutaneous warts: A meta-analysis and pooled analysis of randomized controlled trials. Br J Dermatol. 2011;165:233-46.
- [CrossRef] [PubMed] [Google Scholar]
- Intralesional bleomycin in the treatment of cutaneous warts: A randomized clinical trial comparing it with cryotherapy. Indian J Dermatol Venereol Leprol. 2009;75:262-7.
- [CrossRef] [PubMed] [Google Scholar]
- Bleomycin in dermatology: A review of intralesional applications. Dermatol Surg. 2008;34:1299-313.
- [CrossRef] [PubMed] [Google Scholar]
- Intralesional drug therapy in dermatology. Indian J Dermatol Venereol Leprol. 2017;83:127-32.
- [CrossRef] [PubMed] [Google Scholar]
- Our experience in using bleomycin injection for resistant viral wart treatment. J Am Acad Dermatol. 2015;72(5 Suppl 1):AB211.
- [CrossRef] [Google Scholar]
- Ungual warts: Comparison of treatment with intralesional bleomycin and electroporation in terms of efficacy and safety. J EurAcad Dermatol Venereol. 2019;33:2349-54.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment of verruca vulgaris in both external auditory canals using bleomycin injections. Clin Exp Otorhinolaryngol. 2015;8:295-7.
- [CrossRef] [PubMed] [Google Scholar]
- Impact of multiple extragenital warts on quality of life in immune-competent Egyptian adults: A comparative cross-sectional study. Clin Cosmet Investig Dermatol. 2018;11:289-95.
- [CrossRef] [PubMed] [Google Scholar]
- The treatment of keloids and hypertrophic scars with intralesional bleomycin in skin of color. J Cosmet Dermatol. 2015;14:83-90.
- [CrossRef] [PubMed] [Google Scholar]
- Intralesional bleomycin sulfate injection for the treatment of verruca plantaris. J Am Podiatr Med Assoc. 2006;96:220-5.
- [CrossRef] [PubMed] [Google Scholar]
- Intralesional bleomycin for warts: Patient satisfaction and treatment outcomes. J Cutan Med Surg. 2015;19:470-6.
- [CrossRef] [PubMed] [Google Scholar]
- Intralesional bleomycin injection vs microneedling-assisted topical bleomycin spraying in treatment of plantar warts. J Cosmet Dermatol. 2019;18:124-8.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of intralesional injection of bleomycin in the treatment of plantar warts: Clinical and dermoscopic evaluation. Int J Dermatol. 2018;57:1533-7.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy of intralesional bleomycin in the treatment of resistant warts. J Skin Stem Cell. 2014;1:e18875.
- [CrossRef] [Google Scholar]
- Efficacy of intralesional bleomycin in palmo-plantar and periungual warts. J Cutan Aesthet Surg. 2011;4:188-91.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of efficacy and safety of intralesional bleomycin in the treatment of common warts: Results of a pilot study. Indian J Dermatol Venereol Leprol. 2019;85:397-404.
- [CrossRef] [PubMed] [Google Scholar]
- Intralesional bleomycin for the treatment of resistant palmoplantar and periungual warts. Dermatol Res Pract. 2021;2021:8655004.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy and safety of intralesional bleomycin in the management of ungual warts. Skin Appendage Disord. 2020;6:346-50.
- [CrossRef] [PubMed] [Google Scholar]
- Intralesional bleomycin and Raynaud's phenomenon. J Am Acad Dermatol. 1991;24(5 Pt 1):785-6.
- [CrossRef] [PubMed] [Google Scholar]
- Raynaud phenomenon after treatment of verruca vulgaris of the sole with intralesional injection of bleomycin. Pediatr Dermatol. 2001;18:249-51.
- [CrossRef] [PubMed] [Google Scholar]






