Translate this page into:
Anti-nuclear antibodies: A practical approach to testing and interpretation
-
Received: ,
Accepted: ,
How to cite this article: Santhosh P, Ajithkumar K. Anti-nuclear antibodies: A practical approach to testing and interpretation. J Skin Sex Transm Dis 2021;3:175-9.
Abstract
Anti-nuclear antibodies (ANAs) are a group of antibodies that are characteristically associated with connective tissue diseases (CTDs). Indirect immunofluorescence antibody technique, having a high sensitivity, is the most common technique used for detection, results of which are expressed in terms of the pattern of fluorescence, substrate used, and the titer of a positive test. Other methods include solid-phase assays. ANA test must be performed only when there is a clinical suspicion of an autoimmune CTD. ANA should not be used as a screening tool for asymptomatic individuals. It is essential in clinical practice to be aware of when to order ANA testing, and how to correctly interpret the test results.
Keywords
Antinuclear antibodies
Connective tissue diseases
Systemic lupus Erythematosus
Indirect immunofluorescence
INTRODUCTION
Anti-nuclear antibodies (ANAs) are a group of antibodies that target macromolecules which bind DNA, RNA, proteins, and their complexes.[1,2] Although they bind cytoplasmic components too, the term ANA was retained for historical reasons.[3] Characteristically associated with connective tissue diseases (CTD), ANA may also be detected in other conditions and even in general population.[4]
HISTORY
LE cell phenomenon was described by Hargraves, Richmond, and Morton in 1948.[5] Kidney or liver sections from rats or mice were used as substrates initially to detect ANAs with indirect immunofluorescence antibody (IFA) technique, which are now replaced with human epithelial type-2 (HEp-2) cells.[6-8]
Techniques to detect ANA
The methods to detect ANA can be broadly classified as indirect immunofluorescence technique and solid-phase assays.
Indirect IFA technique/fluorescent ANA (FANA)
The method of choice for the detection of ANA is the indirect IFA technique, otherwise known as FANA technique.[2,9-11] It involves incubation of serum or plasma with either a tissue section or a cell line fixed to a glass slide. Large nuclei of HEp-2 cells make the detection of fluorescence staining patterns easier. Serial dilutions of positive samples are tested to obtain an endpoint titer, and dilution prior to this endpoint is reported as ANA titer.[1,2,4,6] Three parameters are evaluated – the pattern of fluorescence, substrate used, and the titer of a positive test [Figure 1].[4]
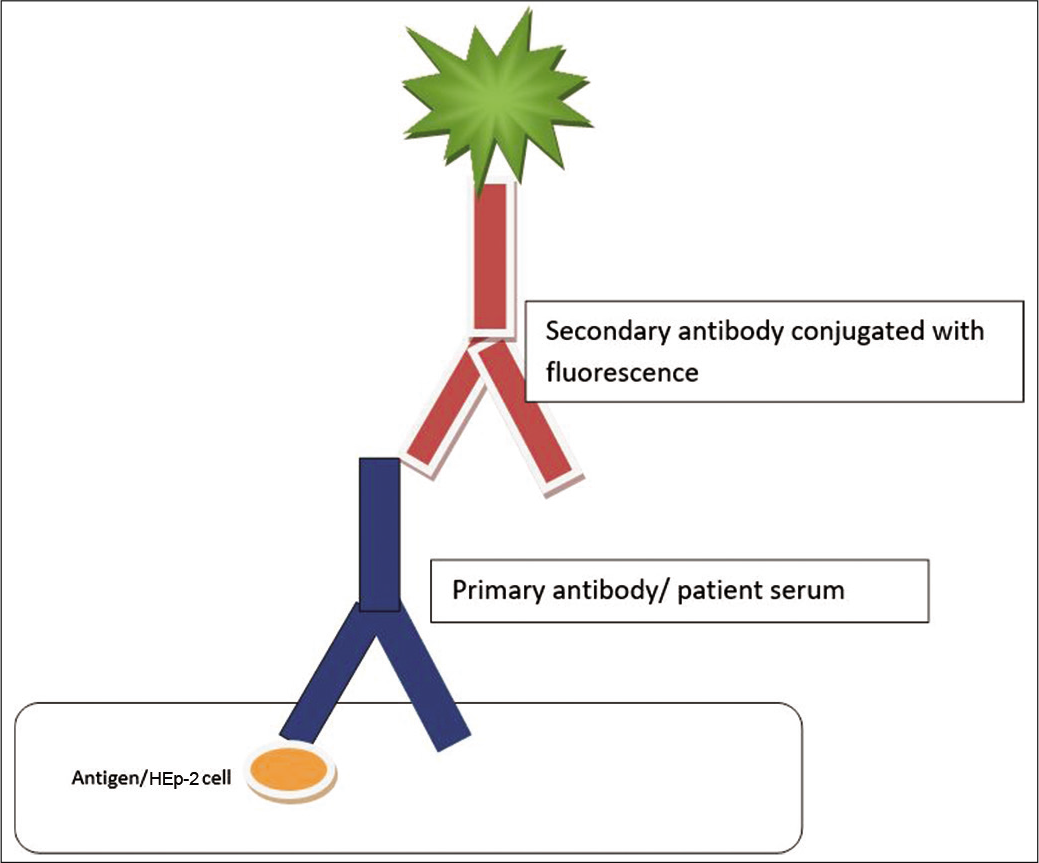
- Diagrammatic representation of the detection of antinuclear antibody using immunofluorescence technique.
Interpretation of ANA titer by immunofluorescence
ANA titer is the quantitative expression of ANA concentration, in dilution. There is a lack of consensus regarding recommended initial dilution for screening purpose, although a titer of 1:160 is taken as significant.[4] Lower titers are also suggested by some experts.[5,6,10-13]
STAINING PATTERNS
The staining patterns of ANA on HEp-2 cells can be broadly divided into nuclear, cytoplasmic, and mitotic patterns [Tables 1 and 2].[14] The intensity of staining expressed in qualitative scale + to ++++ should also be reported, as fluorescence intensity is considered proportional to antibody concentration, which may predict the severity of the CTD. Although staining patterns may provide a clue to the underlying CTD, they cannot be considered specific.[4,14]
| Patterns | Subtypes |
|---|---|
| Nuclear patterns | Homogeneous Speckled (fine and coarse) Peripheral/rim Nucleolar Centromeric PCNA Nuclear dots Nuclear membrane Diffuse grainy |
| Cytoplasmic patterns | Speckled Mitochondrial-like Ribosomal-like Golgi apparatus Lysosomal-like Cytoskeletal filaments (actin, vimentin, and cytokeratin) |
| Mitotic patterns | Mitotic spindle Centrosomes NuMA mid-body CENP-F |
IFA: Indirect immunofluorescence antibody test, PCNA: Proliferating cell nuclear antigen, NuMA: Nuclear mitotic apparatus, CENP-F: Centromere protein F precursor
| ANA pattern | Antigen | Associated diseases |
|---|---|---|
| Homogenous/diffuse | dsDNA, nucleosomes, histones | SLE, juvenile idiopathic arthritis, drug-induced LE |
| Speckled/granular | ENA, RNP, Sm, SSA/Ro, SSB/La, Scl-70, Jo-1, ribosomal-P | SLE, mixed CTD, systemic sclerosis, primary Sjogren’s syndrome, polymyositis |
| Peripheral | RNP, Sm, Ro/SSA | SLE, systemic sclerosis |
| Nucleolar | Anti-PM-Scl, anti-RNA polymerase I-III, anti-U3-RNP | Systemic sclerosis, polymyositis |
| Centromere | CENP A-E | Limited cutaneous systemic sclerosis |
IF-ANA: Immunofluorescence antinuclear antibody, SLE: Systemic lupus erythematosus, LE: Lupus erythematosus, CTD: Connective tissue disease, ENA: Extractable nuclear antigen, DNA: Deoxyribonucleic acid, RNP: Ribonucleoprotein, RNA: Ribonucleic acid, PM: Polymyositis, CTD: Connective tissue disease, CENP: Centromere protein
Advantages of IFA technique for ANA
Disadvantages
The specificity is low – approximately 43% at 1:40 dilution and 63% at 1:80 dilution[15]
The test can be affected by many variables, such as the quality of ingredients, the specificity of substrate, the conjugate, microscope bulb, and reader[11,16]
HEp-2 cells may lack some antigens, such as the Ro-60 antigen and the ribosomal-P[17]
It is labor and skill intensive.
SOLID-PHASE ASSAYS
A panel of purified autoantigens is prepared, and each antigen is immobilized on a solid surface (microtiter plate, fluorescent microsphere, or membrane). Diluted human serum is incubated with the immobilized antigen and a secondary antibody is used to detect bound autoantibodies. These include:
Enzyme-linked immunosorbent assay [Figure 2]
Immunoblot assay
Line immunoassay
Chemiluminescence immunoassays
Multiplex bead-based assays
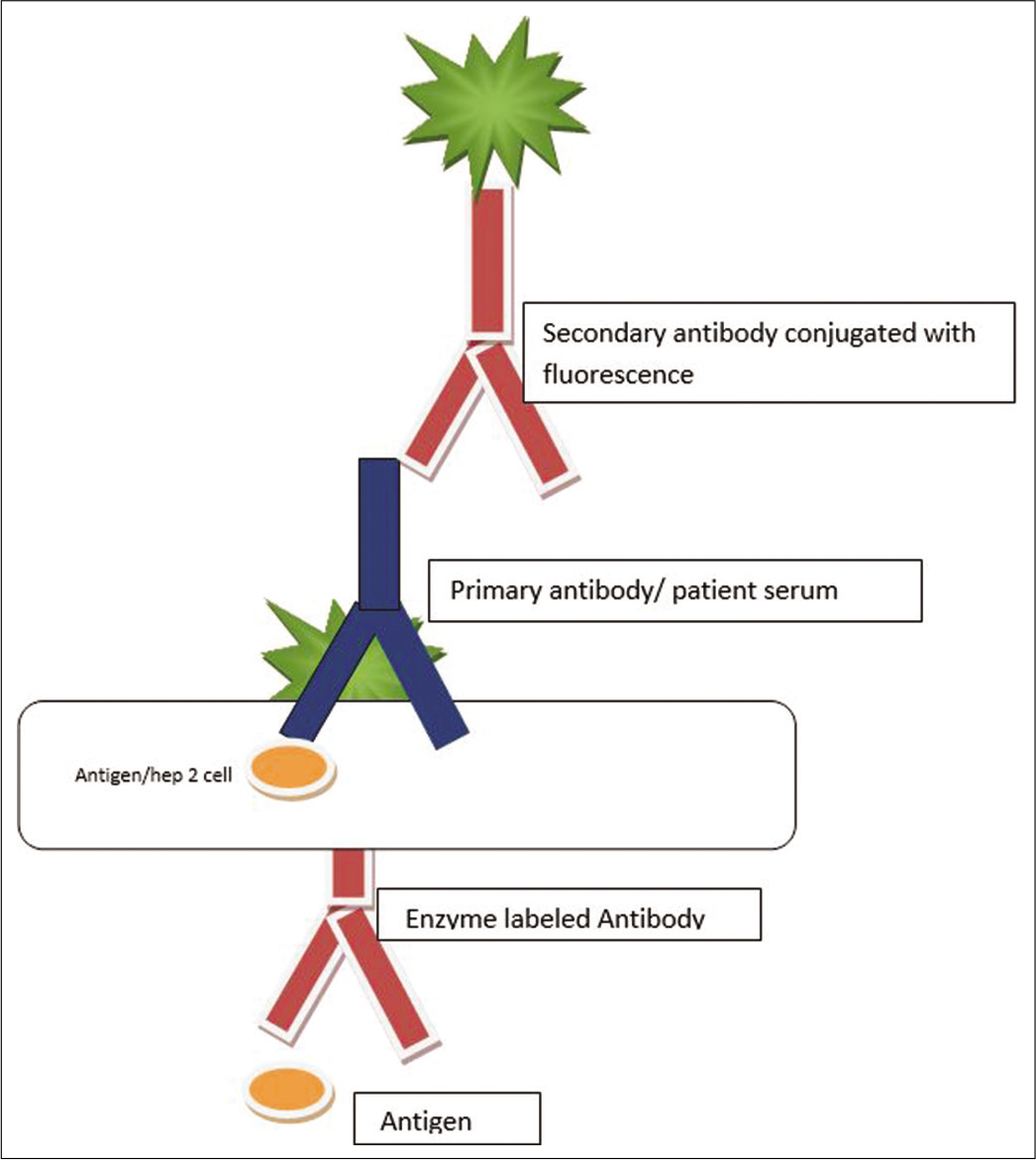
- Diagrammatic representation of the detection of antinuclear antibody using enzyme-linked immunosorbent assay technique.
Advantages of solid-phase assays
They are suitable for high throughput testing
Semi-quantification of results is possible
Automation can increase efficiency and decrease labor cost
Simultaneous identification of the responsible autoantibody.[11]
Disadvantages of solid-phase assays
ANA profile
Many laboratories provide a bouquet of antibody tests in addition to FANA. The components of this panel can be different in different laboratories, but usually include antibodies to single-stranded and double-stranded DNA, ribonucleoprotein antigens, Ro, La, Sm, and topoisomerase I (Scl-70) antigens. ANA profile has higher sensitivity than FANA. ANA profile can also provide additional diagnostic and prognostic information depending on the type of antibody present. Different laboratories use different techniques for the performance of ANA profile, and unfortunately, the performance of many of these tests is not consistent always.[18]
Fallacies of ANA testing
Although rheumatic diseases are quite uncommon in general population, the frequency of ANA positivity determined by IFA in an otherwise healthy population can be high. Most people who are ANA positive will never develop a rheumatic disease.[1]
Just like any other test, the higher the pre-test probability that a patient has a rheumatological disease, the more likely that an ANA test will be of use in establishing the diagnosis. Hence, ANA should not be used as a screening tool for asymptomatic individuals. Indiscriminate testing will cause positive results in 5% at the predetermined screening dilution (usually 1:160).[1] A positive ANA test is important only in conjunction with clinical evaluation. A positive result of ANA or diagnosis of SLE should not lead to all clinical features being interpreted as manifestations of SLE (Greenwald’s law of lupus).[4]
However, screening of patients could be valuable for identifying individuals prone to develop SLE who have a pre-autoimmunity state, where abnormalities of the immune system cause autoreactivity, but clinical disease is yet to manifest.[1,19,20] The titer and binding pattern of ANAs through IFA and the identification of specific ANAs such as anti-dsDNA, anti-Ro60, or anti-La antibodies may be helpful in such situations.[1,17,21]
Conditions other than CTDs which cause positive ANA
A number of non-rheumatological conditions can yield a positive ANA test [Table 3].[22]
| Normal population | |
|---|---|
| Liver disease | Chronic hepatitis Primary cirrhosis Autoimmune hepatitis |
| Infections | Tuberculosis Viral hepatitis Parasitic diseases Syphilis |
| Skin diseases | Psoriasis Lichen planus |
| Malignancies | Breast cancer Prostate cancer Hodgkin’s lymphoma Leukemia |
| Other autoimmune diseases | Type 1 diabetes Addison’s disease Autoimmune anemia Hashimoto’s thyroiditis |
ANA testing: Which test to order first
The American College of Rheumatology ANA task force in 2009 recommended that the immunofluorescence test should remain the gold standard for ANA testing, due to high sensitivity (>95%).[23,24]
When to test for ANA
ANA test must be performed only when there is a clinical suspicion of an autoimmune CTD. If screening test for ANA is positive, further tests for specific autoantibodies must be ordered, based on the clinical features and possible diagnosis. If ANA screening is negative, patient must be reevaluated and kept under follow-up, and test must be repeated only if clinically indicated.[4] If ANA is negative in a patient with a high degree of clinical suspicion for SLE, ANA may be repeated. If negative again, anti-SSA antibody may assist in diagnosis of ANA-negative SLE.[4,25]
When not to test for ANA
For confirming rheumatoid arthritis or osteoarthritis, as it is not considered helpful
To evaluate fatigue, back pain, or other musculoskeletal pain, unless they are accompanied by clinical features suggestive of a CTD
For screening of asymptomatic individuals
ANA tests do not need to be repeated, as changes in ANA titer are not of value in monitoring disease activity
Negative tests need to be repeated only if there is a strong suspicion of an evolving CTD, or a change in the patient’s illness that suggests a revision in diagnosis.[4]
ANA-negative lupus
Several cases of ANA negative lupus have been published, and its prevalence may be as high as 5%–10%.[26] The causes of ANA-negative lupus include antigen-deficient substrate and leaching of antigens, concurrent immunosuppressive treatment, persistent renal loss of proteins, and a false diagnosis of lupus.[27]
CONCLUSION
ANA testing is an important prong in the diagnosis of rheumatological diseases, but in itself has no value in the absence of clinical correlation. A standalone positive or negative ANA test offers nothing to the patient or clinician, and hence, an awareness regarding the judicious use of the same is imperative in clinical practice.
Declaration of patient consent
Not required as there are no patients in this article.
Financial support and sponsorship
Nil.
Conflicts of interest
Dr Kidangazhiathmana Ajithkumar is on the editorial board of the Journal.
References
- Antinuclear antibody testing-misunderstood or misbegotten? Nat Rev Rheumatol. 2017;13:495-502.
- [CrossRef] [PubMed] [Google Scholar]
- Recent approaches to optimize laboratory assessment of antinuclear antibodies. Clin Vaccine Immunol. 2017;24:e00270-17.
- [CrossRef] [PubMed] [Google Scholar]
- Antibodies to cytoplasmic antigens in lupus erythematosus, Serologic marker for systemic disease. Arthritis Rheum. 1977;20:1457-63.
- [CrossRef] [PubMed] [Google Scholar]
- Antinuclear antibodies and their detection methods in diagnosis of connective tissue diseases: A journey revisited. Diagn Pathol. 2009;4:1.
- [CrossRef] [PubMed] [Google Scholar]
- Presentation of two bone marrow elements; the tart cell and the L.E. cell. Proc Staff Meet Mayo Clin. 1948;23:25-8.
- [Google Scholar]
- Significance of nuclear immunofluorescent patterns. Ann Rheum Dis. 1969;28:313-9.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical comparison of cultured human epithelial cells and rat liver as substrates for the fluorescent antinuclear antibody test. J Rheumatol. 1985;12:265-9.
- [Google Scholar]
- The prevalence of antinuclear antibodies in healthy young persons and adults, comparing rat liver tissue sections with HEp-2 cells as antigen substrate. Clin Exp Rheumatol. 1994;12:137-41.
- [Google Scholar]
- Range of antinuclear antibodies in “healthy” individuals. Arthritis Rheum. 1997;40:1601-11.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical interpretation of antinuclear antibody tests in systemic rheumatic diseases. Mod Rheumatol. 2009;19:219-28.
- [CrossRef] [PubMed] [Google Scholar]
- Antinuclear antibody testing: Methods, indications, and interpretation. Lab Med. 2003;34:113-7.
- [CrossRef] [Google Scholar]
- Antinuclear antibodies by indirect immunofluorescence: Optimum screening dilution for diagnosis of systemic lupus erythematosus. Indian J Med Res. 2007;126:34-8.
- [Google Scholar]
- Standardizing initial dilution titers of antinuclear antibodies for the screening of systemic lupus erythematosus. Indian J Rheumatol. 2019;14:211-7.
- [CrossRef] [Google Scholar]
- Report of the first international consensus on standardized nomenclature of antinuclear antibody HEp-2 cell patterns 2014-2015. Front Immunol. 2015;6:412.
- [CrossRef] [PubMed] [Google Scholar]
- Utility of antinuclear antibody screening by various methods in a clinical laboratory patient cohort. J Appl Lab Med. 2016;1:36-46.
- [CrossRef] [Google Scholar]
- Enzyme-linked immunosorbent assay screening then indirect immunofluorescence confirmation of antinuclear antibodies: A statistical analysis. Am J Clin Pathol. 2011;135:678-84.
- [CrossRef] [PubMed] [Google Scholar]
- Limited reliability of the indirect immunofluorescence technique for the detection of anti-Rib-P antibodies. Arthritis Res Ther. 2008;10:R131.
- [CrossRef] [PubMed] [Google Scholar]
- Autoantibody profile testing by immunodiffusion (ID) and hemagglutination (HA): Comparison with routine immunofluorescent antinuclear antibody (FANA) testing. Arthritis Rheum. 1991;34:8.
- [Google Scholar]
- Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med. 2003;349:1526-33.
- [CrossRef] [PubMed] [Google Scholar]
- Early events in lupus humoral autoimmunity suggest initiation through molecular mimicry. Nat Med. 2005;11:85-9.
- [CrossRef] [PubMed] [Google Scholar]
- Pattern on the antinuclear antibody-HEp-2 test is a critical parameter for discriminating antinuclear antibody-positive healthy individuals and patients with autoimmune rheumatic diseases. Arthritis Rheum. 2011;63:191-200.
- [CrossRef] [PubMed] [Google Scholar]
- Antinuclear antibodies in healthy people and non-rheumatic diseases-diagnostic and clinical implications. Reumatologia. 2018;56:243-8.
- [CrossRef] [PubMed] [Google Scholar]
- ANA screening: An old test with new recommendations. Ann Rheum Dis. 2010;69:1420-2.
- [CrossRef] [PubMed] [Google Scholar]
- International recommendations for the assessment of autoantibodies to cellular antigens referred to as anti-nuclear antibodies. Ann Rheum Dis. 2014;73:17-23.
- [CrossRef] [PubMed] [Google Scholar]
- Guidelines for clinical use of the antinuclear antibody test and tests for specific autoantibodies to nuclear antigens, American College of Pathologists. Arch Pathol Lab Med. 2000;124:71-81.
- [Google Scholar]
- Systemic lupus erythematosus presenting as chronic serositis with no demonstrable antinuclear antibodies. Am J Med. 1984;76:1100-5.
- [CrossRef] [Google Scholar]
- Paradigm shift in antinuclear antibody negative lupus: Current evidence. Indian J Dermatol Venereol Leprol. 2018;84:384-7.
- [CrossRef] [PubMed] [Google Scholar]






