Translate this page into:
Basal cell carcinoma: Epidemiology
*Corresponding author: Reshmi Gangan, Department of Dermatology, Primary Health Care Corporation, Doha, Qatar. reshgang@yahoo.co.in
-
Received: ,
Accepted: ,
How to cite this article: Gangan R. Basal cell carcinoma: Epidemiology. J Skin Sex Transm Dis 2022;4:157-63.
Abstract
Basal cell carcinoma is the most common skin cancer in white skinned individuals with a rising incidence observed worldwide. The underlying etiopathogenesis is complex and involves an interplay between ultraviolet radiation, phenotype, and genotype. This review discusses the incidence and the phenotypical and environmental risk factors associated with basal cell carcinoma.
Keywords
Basal cell carcinoma
Ultraviolet radiation
Phenotype
Risk factor
INTRODUCTION
Carcinomas arising from the keratinocytes are the most common cancers worldwide.[1] The exact proportion of skin cancers constituted by squamous cell carcinoma (SCC) and basal cell carcinoma (BCC) remains unclear since many cancer registries document the diagnosis as nonmelanoma skin cancer, instead of recording the exact type of the neoplasm.[2]
INCIDENCE
As per earlier studies, BCC contributed to most of the keratinocyte carcinomas. Recent years have seen a rise in the incidence of both SCC and BCC. This increase is more pronounced for SCC.[1] A study from Minnesota reported an increase by 145% in the overall incidence of BCC during 2000–2010, in comparison to 1976–1984. During the same period, a greater rise was noted in the incidence of SCC (263%).[3] This is attributed partly to the increase in the life span and the effect of chronic ultraviolet (UV) exposure in the elderly. SCC is more common in elderly, while BCC is more often seen in relatively younger age groups.[1] Although a more marked rise is noted in the incidence of SCC, BCC still remains the major non-melanoma skin cancer worldwide with an incidence rate twice that of SCC.[1] However, variations are noted in different population groups.[3]
A systematic review that analyzed the data from 38 different countries worldwide over the period 1955–2007 found the highest annual incidence rate for BCC in Australia (>1000/100,000 person-years) and the lowest rates in parts of Africa (<1/100,000 person-years).[4]
A population-based study in Olmsted County, Minnesota (2000–2010) recorded the incidence of BCC as 360/100,000 persons for men and 292.9 for women.[3] European age-standardized incidence rate for BCC as documented in United Kingdom in 2013–2015 was 285/100,000 person-years.[5] Pandeya et al., in a study, aimed to assess the incidence and multiplicity of BCC and SCC excised in Australia, reported an age-standardized incidence rate of 770/100,000 person-years for BCC (after analyzing individual-level Medicare data for keratinocyte cancer treatments during 2011–2014).[6]
Non-melanoma skin cancers are rare in Asians, probably due to the protective effect of melanin against the deleterious effects of UV light.[7] Incidence rate of BCC in colored skin ranged from 1.5 to 15.57/100,000 population in previous studies.[8,9]
No data are available on the annual incidence rate of BCC in India.[7] Various Indian studies have reported SCC to be more frequent than BCC, which were consistent with the findings in other dark skinned populations.[10,11]
A study from Singapore noted age-standardized incidence rates of 6.9, 2.6, and 1.4/100,000 person-years for BCC among the Chinese, Malays, and Indians, respectively, in 2016.[12] The same study found that the age-standardized incidence rates for BCC were 2.7, 1.2, and 0.5 tumors/100,000 person-years, respectively, among the Chinese, Malays, and Indians in 1968, showing a clear increase in incidence, which was more pronounced for the Chinese.[12]
PATHOGENESIS
Nearly, all BCC show constitutive activation of Hedgehog signaling pathway [Flow Chart 1], which plays a major role in the maintenance of cutaneous stem cells and development of sebaceous glands and hair follicles.[1] In BCC, activation of hedgehog signaling pathway is mostly observed due to mutations that inactivate protein patched homolog 1 gene (located on chromosome 9q) or activate Smoothened gene (located on chromosome 7q). Less frequently, loss-of-function mutations in suppressed fusion (SUFU) protein are seen in BCC. SUFU acts as a negative regulator of hedgehog pathway.
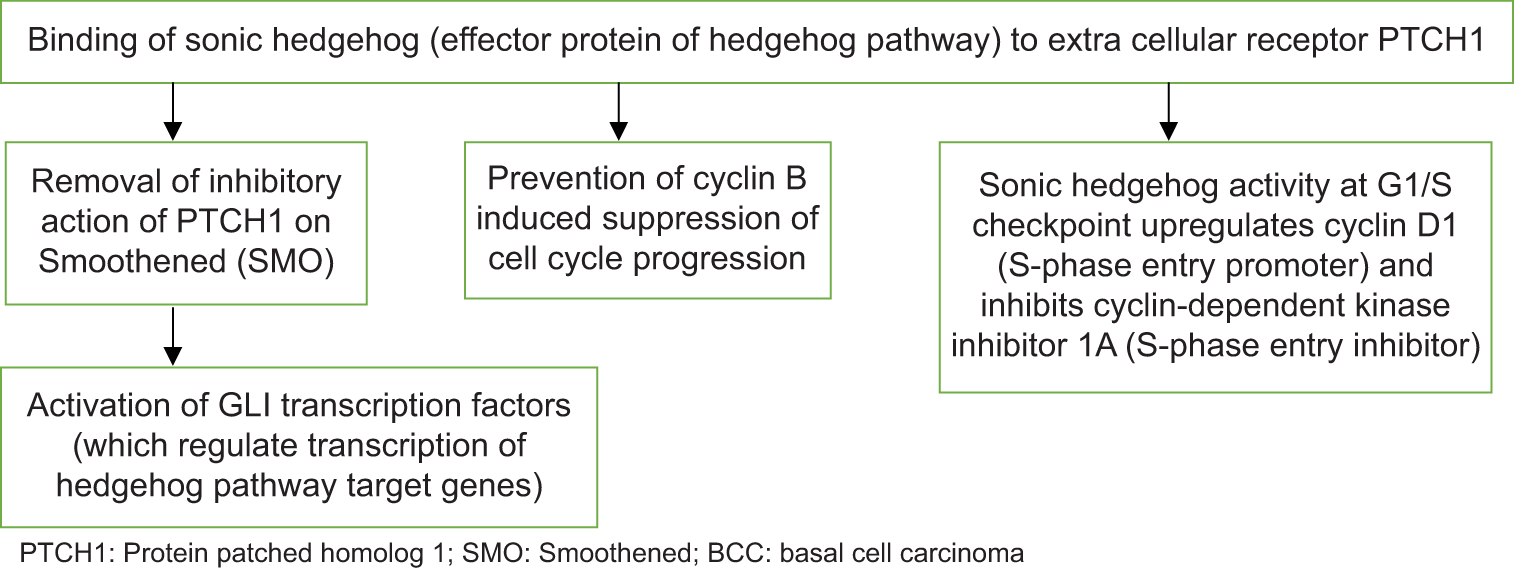
- Hedgehog signaling pathway in the pathogenesis of BCC.
An important environmental risk factor that can predispose to BCC is UV radiation (UVR).[13] The sources of UVR include sunlight, indoor tanning (tanning beds and tanning lamps), UV phototherapy, and arc welding. UVR can induce carcinogenesis by production of deoxyribonucleic acid (DNA) damage, activation of different oncogenes, and inhibition of tumor suppressor genes. UVR-induced gene mutations promote survival and proliferation of keratinocytes. UVR-induced chronic inflammation also contributes to the tumor formation.[13]
The following section focuses on the risk factors for BCC.
RISK FACTORS
Sunlight
Sunlight, predominantly UVB is considered as the most important risk factor for BCC and the risk correlates with the amount and nature of accumulated exposure.[1,14] UV exposure leads to BCC by inducing mutations in coding DNA of keratinocyte progenitor cells of interfollicular epidermis and upper infundibulum. It is reported that, on most occasions, the mutation leads to cytobutane dimer formation.[1] Among cancers affecting humans, BCC shows the highest number of mutations.[1] This could be attributed to the universal presence of the primary cause (UV light) for mutagenesis.[1]
A higher incidence of BCC observed among Asians living closer to the equator suggests that UV exposure is the major risk factor for BCC in Asian skin as well.[1]
Intermittent, intense sun exposure such as recreational tanning is more likely to produce BCC, unlike SCC which is more commonly associated with chronic and cumulative sun exposure.[1]
Studies have shown association between number of sunburns and BCC, especially sunburns sustained during early or middle life.[15,16] However, certain studies have reported chronic UV exposure also as a risk factor for BCC.[17,18]
A meta-analysis of 24 studies documented an association between outdoor work and BCC with a more marked association noted in studies conducted in high latitude countries.[19]
Fair skin, red or blond hair, light eye color, an inability to tan, and a tendency to freckle potentiate the risk for BCC.[1] It has been reported that Asians with lighter skin tone are more likely to receive a diagnosis of BCC than those with darker skin tones.[20]
Signs of photodamage (melanocytic naevi, freckles, solar elastosis, solar lentigines, and actinic keratoses) predict an increased risk for BCC.[21]
The use of tanning beds is documented as a risk factor for skin cancers, more so for BCC. A higher risk is observed with younger age at exposure.[22]
Age and gender predilection
Advancing age is an independent risk factor for BCC with a doubling in incidence from 40 to 70 years.[4] The reduced ability to repair the UVR-induced DNA damage with advancing age is considered as the reason for the higher incidence of BCC in older individuals.[21]
Mean age of the affected varied from 60 to 65 years in most of the studies, but a mean age of 70.1 years was documented in a study on 11,548 patients with BCC, which gathered the data from the Polish National Health Fund database (1999–2019).[2] However, recent years have witnessed a rise in incidence of BCC among those below 40 years.[2]
The gender predilection varied in different studies [Table 1].[2,3,9,23-26] Among patients below 40 years, the incidence of BCC was higher in women even in studies that reported similar incidence rates among males and females.[3] Higher use of tanning beds by young women and a closer attention to appearance by women of young age are cited as the reasons for the female predominance noted in those below 40 years.[21] On the contrary, in old age, the disease showed a clear male predilection.[3]
| Study | Study participants, number of study participants, study setting | Mean age | Male to female ratio | Most common site affected | Most common histological type |
|---|---|---|---|---|---|
| Muzic et al. | 3325 patients with BCC assessed in a population-based study in Olmsted County, Minnesota, that reviewed the medical records of patients who were diagnosed with non-melanoma skin cancer from 2000 to 2010 | 63.4 years | 1:1 | Head and neck | Nodular |
| Al-Qarqaz et al. | 335 cases assessed in a retrospective descriptive study carried out by retrieving BCC cases from the electronic records from the pathology department of a referral hospital in north of Jordan, for the period 2004–2018 | Mean age for males 61.9 years, Mean age for females 65.4 years | 1.76:1 | Face | - |
| Ciążyńska et al. | 11,548 patients with BCC assessed from the data gathered from the Polish National Health Fund database (1999–2019) | 70.1 years | 0.99:1 | Face | |
| Ciążyńska et al. | 890 patients with BCC were assessed in a retrospective study of all patients with BCC who were diagnosed and treated in the dermatology and venereology department of a referral hospital in Poland from 1999 to 2015 | 66.15 years | 0.77:1 | Face (mostly on nose) | Nodular |
| Raina et al. | 46 cases of BCC assessed in a hospital-based observational study which was carried out in the pathology and dermatology departments of a tertiary care center of Himachal Pradesh, India, from January 2012 to December 2017 | 65.7±12.9 years | 1.9:1 | Head and neck (mostly nose) | Solid |
| Malhotra et al. | 34 consecutive cases of clinically diagnosed BCC who attended a referral hospital in North India from January 2007 to December 2009 | 40–60 years | 1.6:1 | Head and neck (mostly medial/ lateral canthus of eye) | Solid-nodular |
| Kumar et al. | 36 patients with BCC assessed in a hospital based cross sectional study in Punjab, North India (2011–2013) | 60.9 years | 0.6:1 | Head and neck (mostly nose) | Nodular |
| George et al. | A retrospective study in 29 patients (with histopathologically confirmed BCC) who attended a referral hospital in the Indian state of Kerala from 2012 to October 2018 | 64.2 years | 0.6:1 | Face (mostly nose) | Superficial spreading variant |
BCC: Basal cell carcinoma
Many Indian studies have noted a female preponderance.[23-26] This is attributed to the long hours spent in sun by women in rural areas, while doing agriculture work and homestead activities, and caring for farm/domestic animals.[23] It was noted that women were intermittently exposed to intense sunlight, while working in open kitchens.[25]
A female predilection is noted for BCC among African Americans also.[14]
Loh et al. reported a female predilection for BCC among Asians and Hispanics in United States.[27]
Diseases and treatment modalities
An early-onset (in childhood) BCC is associated with genetic diseases such as basal cell nevus syndrome, xeroderma pigmentosum, nevus sebaceous, and epidermodysplasia verruciformis.[1] Other genetic diseases that can predispose to BCC are Bazex–Dupre–Christol syndrome, Rombo syndromes, Rothmund–Thomson syndrome, Werner syndrome, Bloom syndrome, and Muir–Torre syndrome.[28]
There are reports of BCC developing on lesions of lichen planus, lupus vulgaris, and verrucous epidermal nevus.[29-31]
Rheumatoid arthritis is reported to be an independent risk factor for BCC.[1] Evidence points to a protective role for other autoimmune diseases such as vitiligo and alopecia areata.[1]
Incidence of BCC among organ transplant recipients is documented to be about 7–16 times higher than the same in general population.[1,21]
A systematic review on people with human immunodeficiency virus (HIV) infection aged more than 50 years showed no significant association between the infection and the keratinocyte carcinomas.[32] A Danish study found a higher risk for BCC and SCC among patients with HIV infection. The higher risk for BCC was noted only in HIV-infected males who engaged in homosexual practices.[33]
Ionizing radiation (younger age at radiation increases the risk) and psoralen and UVA light phototherapy are treatment modalities that can increase the risk of BCC.[1] UVB therapy (>300 treatments) also causes a modest rise in risk of developing BCC.[21] Oral contraceptives are reported to increase the risk for BCC and SCC.[1] The use of oral contraceptives is cited as one reason for the rise in incidence of BCC noted in women.[1] Oral contraceptives and combination hormone replacement therapy showed an association with more aggressive types of BCC.[34] Previous studies have reported an association between photosensitizing agents such as diuretics, tetracyclines, and nonsteroidal anti-inflammatory drugs and BCC.[35-37] However, convincing evidence regarding the role of these drugs as risk factors for BCC is lacking.[21]
Diet
Laboratory analysis favored the theory that dietary antioxidants have the potential to prevent free radical-mediated DNA damage and UVR-induced tumor formation.[38] The role of antioxidants in prevention of non-melanoma skin cancer was evaluated in different randomized controlled trials.[38] Randomized controlled trials that evaluated the role of beta-carotene, selenium, and various combinations of vitamin C, vitamin E, beta-carotene, selenium, and other substances did not find antioxidants to be effective in the prevention of non-melanoma skin cancers.[39-42] Further, it was suggested that beta-carotene supplements may result in exacerbation of UV-induced carcinogenesis.[40,41,43] It was explained that beta-carotene exerts antioxidant effects with the help of a newly created beta-carotene radical cation that contains an unpaired electron. This unpaired electron is strongly oxidizing. It was, further, postulated that beta-carotenes exert photoprotective effect only when combined with other dietary factors.[38] Increased intake of citrus products is known to increase the risk of BCC as the former contain furocoumarins, which show high absorbance of UVR.[13]
Thus, the focus was shifted to the role of whole food instead of vitamin supplements in preventing carcinogenesis. It is proposed that in a fruit or a vegetable, different antioxidants are combined in fixed proportions. The resultant net antioxidant effect depends on the specific combination.[44]
An observational study that focused on “combined consumption of foods” found that a “vegetable and fruit pattern diet” reduced the risk of non-melanoma skin cancer in comparison to meat and fat pattern.[45] The current knowledge supports the intake of diet rich in antioxidants to protect against non-melanoma skin cancer.[38]
It has been documented that intake of caffeine may reduce the risk for BCC and multiple BCC.[21] However, the role of caffeine in preventing photocarcinogenesis remains unclear and needs more convincing evidence.[21]
A dose-related relation exists between arsenic contaminated water, food and medicine, and BCC.[46-48]
Others
There are conflicting opinions regarding association between alcohol consumption and BCC.[1] Cigarette smoking is not identified as a risk factor for BCC.[1] The available evidences on association between long-term residential radon exposure and BCC are contradictory.[1]
There is no convincing evidence to support an association between human papillomavirus infection and BCC.[49]
RISK FACTORS FOR RECURRENT AND MULTIPLE BCC
A patient with a history of BCC has a 17-fold higher risk for a second BCC in comparison to general population.[21]
It is reported that patients with a keratinocyte carcinoma (BCC or SCC) often develop another BCC or SCC and different signs of photodamage such as solar keratosis and actinic keratosis.[50] Weinstock proposed the term “actinic neoplasia syndrome” to denote this predisposition due to field dysplasia.[50]
Personal or family history of skin cancer increases the risk for BCC.[21] Verkouteren et al. observed age at first BCC (maximum risk at 68 years), male sex and initial BCC of superficial subtype as risk factors for a second BCC, while coffee consumption reduced the chance of the same.[21] Having more than one initial BCC was the strongest predictor for a second BCC.[21]
Sgouros et al., in a retrospective study on patients with surgically excised and histopathologically diagnosed BCC identified a personal history of BCC as the major predisposing factor for multiple BCC.[51] As per literature, the risk of developing a non-melanoma skin cancer after an initial diagnosis of a cutaneous malignancy is 35% and 50% at 3 years and 5 years, respectively.[52] Sgouros et al. found that more patients with multiple BCC were elderly in comparison to those with single BCC and also noted a slight male predilection for multiple BCC.[51] History of at least one severe sunburn during childhood or adolescence was identified as a risk factor for multiple BCC in later life. The study reported that multiple tumors were not associated with a more aggressive behavior.[51]
Hallaji et al., in their study on 218 patients with histopathologically proven BCC, noted mountainous area of birth, history of BCC, history of radiotherapy, abnormal skin at the tumor site, and pigmented pathologic type to be associated with multiple BCC.[53]
CONCLUSION
With the rising trend noted in BCC, we are expected to see more cases in the coming years. A significant increase noted among young women assumes significance, since a personal history of BCC itself is an independent risk factor for a second BCC or another skin cancer. The strong association noted between sun exposure and BCC indicates the importance of educating the public regarding the potential risk factors and the measures to avoid them. Avoidance of intense sun exposure (especially in early life), regular use of sunscreens, and avoidance of tanning beds can go a long way in the prevention of BCC. The general population should be made aware of the importance of self-examination of skin to ensure an early detection of the neoplasm.
Declaration of patient consent
Not required as there are no patients in this article.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Basal cell carcinoma. Epidemiology; pathophysiology; clinical and histological subtypes; and disease associations. J Am Acad Dermatol. 2019;80:303-17.
- [CrossRef] [PubMed] [Google Scholar]
- The incidence and clinical analysis of non-melanoma skin cancer. Sci Rep. 2021;11:4337.
- [CrossRef] [PubMed] [Google Scholar]
- Incidence and trends of basal cell carcinoma and cutaneous squamous cell carcinoma: A population-based study in Olmstead County, Minnesota, 2000-2010. Mayo Clin Proc. 2017;92:890-8.
- [CrossRef] [PubMed] [Google Scholar]
- A systematic review of worldwide incidence of nonmelanoma skin cancer. Br J Dermatol. 2012;166:1069-80.
- [CrossRef] [PubMed] [Google Scholar]
- Epidemiology of basal and cutaneous squamous cell carcinoma in the U.K. 2013-15: A cohort study. Br J Dermatol. 2019;181:474-82.
- [CrossRef] [PubMed] [Google Scholar]
- The incidence and multiplicity rates of keratinocyte cancers in Australia. Med J Aust. 2017;207:339-43.
- [CrossRef] [PubMed] [Google Scholar]
- Nonmelanoma skin cancer in India: Current scenario. Indian J Dermatol. 2010;55:373-8.
- [CrossRef] [PubMed] [Google Scholar]
- Changing trends of types of skin cancer in Iran. Asian Pac J Cancer Prev. 2015;16:4955-8.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical and Demographic Features of Basal Cell Carcinoma in North Jordan. J Skin Cancer. 2018;2018:2624054.
- [CrossRef] [PubMed] [Google Scholar]
- Squamous cell carcinoma of skin: its incidence and etiopathogenesis in 625 cases. Ind J Cancer. 1970;7:24-33.
- [Google Scholar]
- Trends of cutaneous basal cell carcinoma, squamous cell carcinoma, and melanoma among the Chinese, Malays, and Indians in Singapore from 1968-2016. JAAD Int. 2021;4:39-45.
- [CrossRef] [PubMed] [Google Scholar]
- Ultraviolet radiation and basal cell carcinoma: An environmental perspective. Front Public Health. 2021;9:666528.
- [CrossRef] [PubMed] [Google Scholar]
- Skin cancer in women of color: Epidemiology, pathogenesis and clinical manifestations. Int J Womens Dermatol. 2021;7:127-34.
- [CrossRef] [PubMed] [Google Scholar]
- Skin cancer in a subtropical Australian population: incidence and lack of association with occupation. The Nambour study group. Am J Epidemiol. 1996;144:1034-40.
- [CrossRef] [PubMed] [Google Scholar]
- Risk factors for basal cell carcinoma of the skin in men: results from the health professionals follow-up study. Am J Epidemiol. 1999;150:459-68.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence of actinic skin lesions in patients with basal cell carcinoma of the head: A case-control study. Rev Assoc Med Bras. 2012;58:188-96.
- [CrossRef] [Google Scholar]
- Ultraviolet exposure and risk of melanoma and basal cell carcinoma in Ulm and Dresden, Germany. J Eur Acad Dermatol Venereol. 2015;29:134-42.
- [CrossRef] [PubMed] [Google Scholar]
- Is occupational solar ultraviolet irradiation a relevant risk factor for basal cell carcinoma? A systematic review and meta-analysis of the epidemiological literature. Br J Dermatol. 2011;165:612-25.
- [CrossRef] [PubMed] [Google Scholar]
- Skin cancer in skin of color. J Am Acad Dermatol. 2006;55:741-60.
- [CrossRef] [PubMed] [Google Scholar]
- Epidemiology of basal cell carcinoma: Scholarly review. Br J Dermatol. 2017;177:359-72.
- [CrossRef] [PubMed] [Google Scholar]
- Use of tanning beds and incidence of skin cancer. J Clin Oncol. 2012;30:1588-93.
- [CrossRef] [PubMed] [Google Scholar]
- Basal cell carcinoma: A 6-year clinicopathological study from the SubHimalayan region of North India. CHRISMED J Health Res. 2019;6:254-8.
- [CrossRef] [Google Scholar]
- Basal cell carcinoma in the North Indian population: Clinicopathologic review and immunohistochemical analysis. Indian J Dermatol Venereol Leprol. 2011;77:328-30.
- [CrossRef] [PubMed] [Google Scholar]
- A study of basal cell carcinoma in South Asians for risk factor and clinicopathological characterization: A hospital based study. J Skin Cancer. 2014;2014:173582.
- [CrossRef] [PubMed] [Google Scholar]
- Clinicopathological analysis of basal cell carcinoma a retrospective study. J Skin Sex Transm Dis. 2021;3:51-5.
- [CrossRef] [Google Scholar]
- Prevalence and clinical characteristics of nonmelanoma skin cancers among Hispanic and Asian patients compared with white patients in the United States: A 5-year, single-institution retrospective review. Dermatol Surg. 2016;42:639-45.
- [CrossRef] [PubMed] [Google Scholar]
- Genetic skin diseases predisposing to basal cell carcinoma. Eur J Dermatol. 2012;22:299-309.
- [CrossRef] [PubMed] [Google Scholar]
- Basal cell carcinoma occurring in a lesion of lichen planus: coincidence or causation? Indian J Dermatol Venereol Leprol. 2008;74:662-4.
- [CrossRef] [PubMed] [Google Scholar]
- Basal cell carcinoma in a long-standing case of lupus vulgaris. Indian J Dermatol Venereol Leprol. 1995;61:10910.
- [Google Scholar]
- Basal cell carcinoma developing in verrucous epidermal nevus. Indian J Dermatol Venereol Leprol. 2007;73:1278.
- [CrossRef] [PubMed] [Google Scholar]
- Risk of skin cancers in older persons living with HIV: A systematic review. J Assoc Nurses AIDS Care. 2019;30:80-6.
- [CrossRef] [PubMed] [Google Scholar]
- Risk of skin cancer in patients with HIV: A Danish nationwide cohort study. J Am Acad Dermatol. 2018;79:689-95.
- [CrossRef] [PubMed] [Google Scholar]
- Sex hormones and the risk of keratinocyte cancers among women in the United States: A population-based case-control study. Int J Cancer. 2016;139:300-9.
- [CrossRef] [PubMed] [Google Scholar]
- High-ceiling diuretics are associated with an increased risk of basal cell carcinoma in a population-based follow-up study. Eur J Cancer. 2010;46:2467-72.
- [CrossRef] [PubMed] [Google Scholar]
- Prescription diuretic use and risk of basal cell carcinoma in the nationwide U.S. radiologic technologists cohort. Cancer Epidemiol Biomarkers Prev. 2014;23:1539-45.
- [CrossRef] [PubMed] [Google Scholar]
- Photosensitizing agents and the risk of nonmelanoma skin cancer: A population-based case-control study. J Invest Dermatol. 2013;133:1950-5.
- [CrossRef] [PubMed] [Google Scholar]
- Diet and Skin Cancer: The potential role of dietary antioxidants in nonmelanoma skin cancer prevention. J Skin Cancer. 2015;2015:893149.
- [CrossRef] [PubMed] [Google Scholar]
- A randomized, 12-year primary prevention trial of beta carotene supplementation for nonmelanoma skin cancer in the physicians' health study. Arch Dermatol. 2000;136:179-84.
- [CrossRef] [PubMed] [Google Scholar]
- Selenium supplementation and secondary prevention of nonmelanoma skin cancer in a randomized trial. J Natl Cancer Inst. 2003;95:1477-81.
- [CrossRef] [PubMed] [Google Scholar]
- The nutritional prevention of cancer: 400Mcg per day selenium treatment. Nutr Cancer. 2008;60:155-63.
- [CrossRef] [PubMed] [Google Scholar]
- A clinical trial of beta carotene to prevent basal-cell and squamous-cell cancers of the skin. The Skin Cancer Prevention Study Group. N Engl J Med. 1990;323:789-95.
- [CrossRef] [PubMed] [Google Scholar]
- Antioxidant supplementation increases the risk of skin cancers in women but not in men. J Nutr. 2007;137:2098-105.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of vitamin treatment or supplements with purported antioxidant properties on skin cancer prevention: A meta-analysis of randomized controlled trials. Dermatology. 2011;223:36-44.
- [CrossRef] [PubMed] [Google Scholar]
- Dietary pattern in association with squamous cell carcinoma of the skin: A prospective study. Am J Clin Nutr. 2007;85:1401-8.
- [CrossRef] [PubMed] [Google Scholar]
- Dermatological manifestations of arsenic exposure. J Skin Sex Transm Dis (Article in press)
- [Google Scholar]
- Basal cell carcinoma in chronic arsenicism occurring in Queensland, Australia, after ingestion of an asthma medication. J Am Acad Dermatol. 2000;43:664-9.
- [CrossRef] [PubMed] [Google Scholar]
- Arsenic in drinking water and skin cancers: Cell-type specificity (Taiwan, ROC) Cancer Causes Control. 2001;12:909-16.
- [CrossRef] [PubMed] [Google Scholar]
- Genus beta human papillomaviruses and incidence of basal cell and squamous cell carcinomas of skin: Population based case-control study. BMJ. 2010;341:c2986.
- [CrossRef] [PubMed] [Google Scholar]
- Quality of life in the actinic neoplasia syndrome: The VA topical tretinoin chemoprevention (VATTC) trial. J Am Acad Dermatol. 2009;61:207-15.
- [CrossRef] [PubMed] [Google Scholar]
- Novel insights for patients with multiple basal cell carcinomas and tumors at high-risk for recurrence: Risk factors, clinical morphology, and dermatoscopy. Cancers. 2021;13:3208.
- [CrossRef] [PubMed] [Google Scholar]
- Occurrence of cutaneous basal cell and squamous cell malignancies among those with a prior history of skin cancer. J Invest Dermatol. 1994;102:10S-13.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of risk factors of single basal cell carcinoma with multiple basal cell carcinomas. Indian J Dermatol. 2011;56:398-402.
- [CrossRef] [PubMed] [Google Scholar]






