Translate this page into:
Beyond skincare routines: Follow your gut to healthy skin – A review of the interplay between gut microbiome and skin
*Corresponding author: Sanjana Joy, Department of Dermatology, Amala Institute of Medical Sciences, Thrissur, Kerala, India. sanjanajoykl@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Criton VJ, Joy S. Beyond skincare routines: Follow your gut to healthy skin – A review of the interplay between gut microbiome and skin. J Skin Sex Transm Dis 2024:6:5-12. doi: 10.25259/JSSTD_49_2023
Abstract
The gut microbiome, comprising trillions of microorganisms with diverse genetic material, plays a pivotal role in human health. Its impact on immunity, metabolism, and even psychological well-being has garnered significant attention. This review delves into the evolution of microbiome research, highlighting historic breakthroughs and modern revelations. The gut microbiota’s influence extends beyond digestion, impacting immune system development, hormone regulation, and maintenance of protective barriers. Disturbances in this balance, known as dysbiosis, can disrupt immune responses, fostering harmful bacteria over beneficial ones. Dysbiosis has been linked to various diseases, motivating exploration of the gut-skin axis. Research suggests that the gut microbiome significantly influences dermatological conditions. Imbalances can contribute to skin diseases like atopic dermatitis and psoriasis. Short-chain fatty acids produced by gut bacteria have anti-inflammatory effects and maintain skin health. Gut dysbiosis can lead to systemic inflammation, affecting skin physiology. Ultraviolet (UV) damage and skin aging are also affected by the gut microbiome. Studies reveal that certain Lactobacillus strains, ingested orally, possess anti-inflammatory properties, mitigating UV-induced skin aging. Moreover, supplementation with specific bacteria enhances skin elasticity and hydration. Conditions such as rosacea, hidradenitis suppurativa, and chronic spontaneous urticaria have been linked to gut dysbiosis. Research indicates a potential for treatments that target the gut microbiome, such as fecal microbiota transplantation and probiotics, to alleviate dermatological disorders. In conclusion, the gut microbiome exerts a profound influence on skin health and diseases. Its intricate interactions with the immune system, systemic inflammation, and barrier maintenance underscore its significance. Harnessing these insights could lead to innovative therapies for various skin conditions, paving the way for enhanced well-being.
Keywords
Gastrointestinal microbiome
Dysbiosis
Acne vulgaris
Urticaria
Psoriasis
INTRODUCTION
Three centuries ago, when Antony van Leeuwenhoek looked through his self-made microscope and saw interesting little creatures, “small animalcules a-moving very prettily” in his stool and dental plaque, the scientific world just was not ready for it. They had not yet stumbled on the idea that germs could be responsible for causing diseases.[1] Now, we comprehend the significance of the human microbiome – a dynamic community of over 100 trillion microorganisms with three million genes.[2] Birth shapes this microbiome as microbes surge from zero to a multitude.[3] Notably, the gut has the highest numbers and diversity of microorganisms.[4] This vibrant and fascinating community, which consists of a diverse range of microorganisms such as bacteria, viruses, protozoa, and fungi, along with their genetic material, is known as the gut microbiome.[5] This intricate microbial world encompasses beneficial and harmful entities, evolving symbiotically with humans. The diversity of the human gut microbiome increases from birth to childhood, then stabilizes, and gradually decreases with age. During the early stages of development, host genetics play a massive role. Later, as an individual grows older, environmental factors become the dominant driver of gut microbiota makeup.[6] Furthermore, studies have revealed that gut microbiome composition is related to one’s personality traits, and those with larger social networks have a more diverse microbiota.[7] The most popular phyla include the Bacteroidetes and Firmicutes, followed by Proteobacteria, Fusobacteria, Tenericutes, Actinobacteria, and Verrucomicrobia.[8]
Over the past decade, scientists have been captivated by the interactions between gut microbes and our bodies, exploring how they impact metabolism, immune responses, and neuroendocrine functions. The exploration of our gut microbiota continues, unveiling new insights into our intricate connection with these influential microscopic inhabitants, as is highlighted in this review article.
EVOLUTION OF THE GUT MICROBIOME CONCEPT
The study of the intestinal microbiome has a rich history that dates back to the 1840s, with significant contributions from various scientists. Lionel Smith Beale and Ernst Hallier confirmed microbial presence in our gut and stool.[9] Escherich identified Bacterium coli commune (Escherichia coli) in the feces of healthy babies.[9] Tissier pioneered the use of “good bacteria” (Bacillus acidiparalactici) as a form of therapy for gastrointestinal diseases.[9] Metchnikoff shed light on the remarkable importance of Lactobacillus acidophilus, and this led to the popularity of fermented milk products. Tablets containing lactic acid bacteria also entered the market for treating conditions such as diarrhea, nephritis, and arteriosclerosis.[10] Alfred Nissle discovered a novel strain of E. coli, later named Nissle 1917, which has the potential to combat Enterobacteria. Nissle cultivated and encapsulated it in gelatin capsules and patented this groundbreaking therapy under the name Mutaflor©, which has continued to be used successfully up to the present day.[9] In 1958, fecal enemas were successfully utilized to treat patients with pseudomembranous enterocolitis caused by Clostridioides difficile infection. This marked an early breakthrough in manipulating the microbiota to improve human health.[11] In 2012, the Human Microbiome Project unveiled a surprising fact – the number of bacteria in our bodies outnumber our cells! Then came the pivotal moment in 2013 when fecal microbiota transplantation (FMT) outperformed antibiotics in treating patients with Clostridium difficile infection.[9] This groundbreaking development marked the beginning of a new era. Scientists began exploring ways to replace harmful microbes in our bodies with beneficial ones, and this approach rapidly gained momentum. Overall, these remarkable scientific milestones, starting with Escherich’s discoveries in 1885 and culminating in the modern era of microbiome research, have revolutionized our perspective on the vital role of microbes in our bodies.
FUNCTIONS OF GUT
The gut performs several distinct functions in addition to its primary function of digestion, absorption, and elimination. It is essential for the development of our immune system and serves as the first line of defense against infections. It produces some hormones that regulate appetite, digestion, and the release of insulin. Its barrier function, which regulates the movement of substances and microorganisms, is another crucial feature.[12] It helps control the growth and activity of microorganisms and produces an environment that is ideal for their survival. A balanced gut microbiota is associated with better immunity, digestion, and general health.[4] Disruptions in the digestive, immunological, or endocrine systems within the gut may play a role in the development of numerous diseases.[13]
FUNCTIONS OF GUT MICROBIOTA
The gut’s microbial inhabitants wield remarkable influence over our bodies, a reciprocal partnership essential to health. Microbes such as Akkermansia muciniphila and Lactobacillus plantarum maintain intestinal integrity, while Bacteroides thetaiotaomicron, Faecalibacterium prausnitzii, and others regulate mucus production, which is crucial for maintaining a healthy gut environment.[10] They digest dietary fibers, yield vitamins (B12, folate), and produce short-chain fatty acids (SCFAs) such as acetic, propionic, and butyric acids, which are vital for cellular activities, even acting as natural tumor suppressors.[14] Microbes synthesize bile acids, protect against pathogens, fortify the immune system, and impact bone development through SCFAs.[15] The gut-brain axis serves important roles in coordinating gut activities and connecting emotional centers in the brain with various intestinal functions.[16] SCFAs help maintain the integrity of the blood-brain barrier, and certain products produced by microbes can affect neurological function, mood, and behavior.[16] Microbiota alterations contribute to disease risk and responses to therapies, with the potential for protective compounds and drug development.[1] They play causal roles in diseases, from obesity to autoimmune and neurological disorders, revealing new avenues for health enhancement.
Considering all these remarkable functions and their incredible significance for our health, it is no wonder that the gut microbiota is often referred to as a “superorganism,” or “the last unexplored human organ,” or an invisible organ.[4] It is also associated with functions of various organs, and this has resulted in terms like “gut-brain axis,” gut-lung axis, and “gut-skin axis.”[17] As a result, any imbalance in the gut microbiota can result in a wide range of illnesses in fields as diverse as psychiatry, neurology, dermatology, gastroenterology, pulmonology, cancer, and rheumatology.
DYSBIOSIS
The term dysbiosis refers to changes in the composition or functionality of the gut microbiota compared to what is considered healthy, thereby disrupting the delicate balance. This condition is characterized by a less diverse and less stable microbiota, and harmful bacteria may increase. It can impair both local and systemic immune responses, weaken the protective barriers in our mucosal tissues, and disrupt the signaling of cytokines.[17] In addition, dysbiosis can hinder the colonization of beneficial bacteria while providing a favorable environment for the growth of harmful pathogens. These pathogens can spread to many sites, including the lymph nodes and circulation, and cause an inflammatory reaction that has an impact on both local and systemic sites.[18] These alterations in the gut microbiota have been linked to a wide range of health conditions.
GUT MICROBIOTA AND DERMATOLOGY
About 90 years ago, Stokes and Pillsbury first proposed the relationship between the gut microbiome and skin disease.[11] Unfortunately, this hypothesis could not be explored due to technical limitations. Recent research, however, has shown that modifications in gut microbiota can lead to the development of numerous dermatological conditions such as atopic dermatitis, psoriasis, acne vulgaris, rosacea, hidradenitis suppurativa, and chronic spontaneous urticaria[19] [Table 1].
| Skin condition | Gut microbiome |
|---|---|
| Atopic dermatitis | • Western diet disrupts gut microbiome balance, reducing short-chain fatty acid production. • Reduction in beneficial bacteria (e.g., Faecalibacterium prausnitzii and Bifidobacterium). • Escherichia coli is associated with eczema risk. • Clostridioides difficile linked to atopic disease risk. |
| Psoriasis | • Reduced Akkermansia muciniphila, Bacteroides, Proteobacteria, and Faecalibacterium prausnitzii. • Actinobacteria and Firmicutes increased. • Gut microbial DNA found in the blood of active psoriasis patients. |
| Ultraviolet damage and skin aging | • Lactobacillus strains orally ingested exhibit anti-inflammatory effects and delay UV-induced skin aging. • Lactobacillus johnsonii supplementation reduces hypersensitivity reactions. • Lactobacillus johnsonii La1 preserves healthy skin response to UV radiation. •Oral supplementation with Lactobacillus plantarum improves skin elasticity and hydration and inhibits matrix metalloproteinase-1 expression associated with skin aging. |
| Hydradenitis suppurativa | Greater amounts of Ruminococcus gnavus and Clostridium ramosum. |
| Chronic spontaneous urticaria | • Higher levels of Lactobacillus, Turicibacter, and Lachnobacterium. • Lower levels of Phascolarctobacterium. |
Skin homeostasis is highly affected by the way gut bacteria interact with our immune system. F. prausnitzii and bacteria from the Clostridium clusters IV and XI encourage the growth of regulatory T-cells. On the other hand, segmented filamentous bacteria can cause an increase in proinflammatory cells such as Th17 and Th1.[20] SCFAs, particularly butyrate, mediate a significant part of this interaction. By preventing inflammatory cells from proliferating, migrating, adhering, and producing cytokines, SCFAs can suppress immunological responses. They accomplish this by targeting important signaling pathways, including Nuclear factor- B (NF-B) and histone deacetylase. SCFAs play an essential role in several skin-related processes, such as hair follicle stem cell differentiation and wound healing, by controlling the activation and apoptosis of immune cells. Recent research has also shown that the gut microbiome can directly impact the physiology, pathology, and immune response of the skin. Bacteria and their metabolites can enter the bloodstream when the intestinal barrier is broken, build up in the skin, and disturb the normal equilibrium. Researchers have found intestinal bacterial DNA in the plasma of people with psoriasis, demonstrating a direct connection between the gut microbiota and skin health.[20] Psychological stress can both initiate and worsen existing skin diseases. It could be because the gut microbiota might release neurotransmitters in response to stress, which modulates the skin function through neural pathways, and this is known as the skin-gut-brain axis.[21] In addition, the skin microbiome’s composition is influenced by the gut microbiome. SCFAs have a significant role in determining the types of microorganisms that thrive on the skin. Propionibacterium has antibacterial properties against pathogens like Staphylococcus aureus and is capable of producing SCFAs. In addition, compared to other microorganisms, commensal bacteria such as Propionibacterium acnes and Staphylococcus epidermidis are more adaptive to variations in SCFA levels.[20] Supplementation with Lactobacillus brevis SBC8803 reduced transepidermal water loss, increased corneal hydration, and improved the integrity of the skin barrier.[20]
The gut microbiota influences both innate and adaptive immune systems. Specific types of bacteria in our gut can improve the response of our skin when its protective barrier is compromised. Lactobacillus helveticus lessens the severity of dermatitis and improves the function of the skin barrier. Lactobacillus paracasei helps in the recovery of the skin barrier and reduces signs of inflammation due to irritants.[20] In addition, Lactobacillus reuteri promotes faster wound healing by facilitating the movement of regulatory T-cells to the wounds and clearing away the neutrophils.[20]
In addition, the gut microbiome can affect how T-cells differentiate in response to immunological signals. By administering Lactobacillus casei, researchers observed a decrease in the differentiation of CD8+ T-cells into cells that contribute to skin hypersensitivity reactions. The recruitment of regulatory T-cells to the skin increased at the same time. This immune-modulatory effect resulted in a reduction in skin inflammation caused by cell death and contributed to the restoration of skin balance.[20]
These findings highlight the key role that gut bacteria play in maintaining the equilibrium and health of our skin. It affects how our immune systems react, speeds up the healing of wounds, and helps maintain a healthy balance between regulatory and proinflammatory immune cells, all of which support skin allostasis. An imbalance of gut bacteria known as intestinal dysbiosis can have a deleterious impact on skin function. When harmful bacteria are present in the gut, they can produce metabolites such as free phenol and p-cresol, which can damage the skin barrier’s integrity and impair skin cells’ ability to differentiate. They can also enter the bloodstream, accumulate in the skin, and contribute to reduced skin hydration and impaired keratinization. In addition, intestinal dysbiosis can make the intestinal lining more permeable, which can cause an imbalance between regulatory T-cells and the activation of effector T-cells. This can, in turn, initiate a cycle of chronic systemic inflammation[20] [Figure 1].
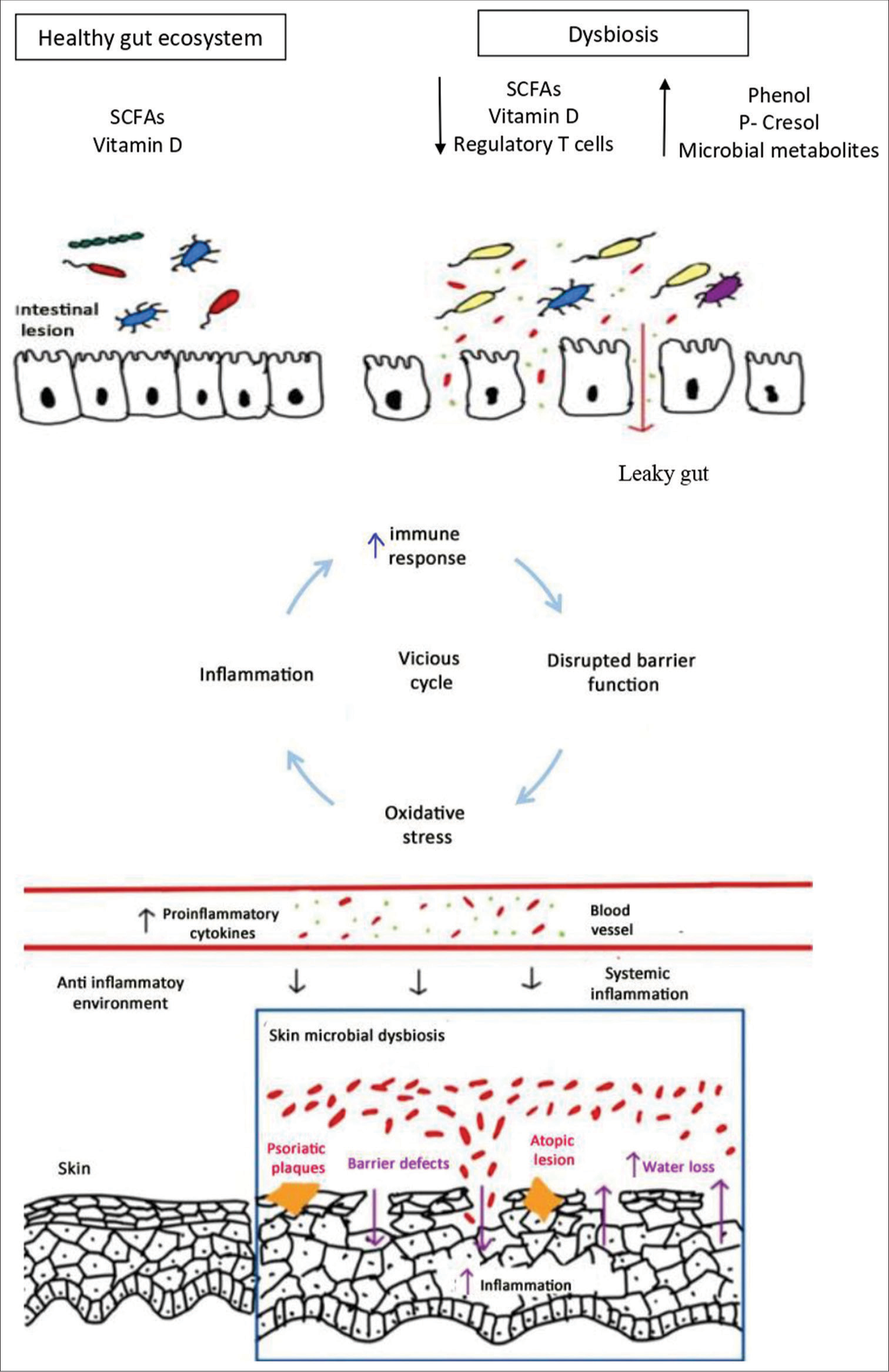
- Gut-skin axis. SCFAs: Short chain fatty acids
Acne vulgaris
Through interactions with the mTOR pathway, the gut microbiota can lead to the development of acne vulgaris. The metabolites produced by the gut microbiota can control mTOR-mediated activities such as cell proliferation and lipid metabolism. Disruptions in the gut barrier and dysbiosis can create a positive feedback cycle of metabolic inflammation, contributing to the underlying processes of acne. Furthermore, there seems to be a potential connection between acne, gastrointestinal dysfunction, and psychological factors. Intestinal and systemic inflammation can result from psychological stressors, including anxiety and depression, which can alter the gut flora and neurotransmitter synthesis. Substance P, a neuropeptide implicated in acne and intestinal dysbiosis, can trigger inflammatory signals that contribute to acne development. Gastrointestinal dysfunction, such as hypochlorhydria, is also associated with acne. It can result in intestinal dysbiosis and an overgrowth of bacteria in the small intestine, known as small intestinal bacterial overgrowth (SIBO). SIBO can lead to the production of toxic metabolites, increased intestinal permeability, and systemic inflammation.[20]
Atopic dermatitis
According to the diet-microbiome theory, the increased occurrence of allergic illnesses, such as atopic dermatitis, is related to a disturbed immunological balance caused by the gut microbiome. The Western diet, characterized by low fiber and high-fat content, can alter the gut microbiome and reduce the production of immunomodulatory metabolites, particularly SCFAs. The reduction in SCFA production and the resulting decrease in immune tolerance mediated by regulatory T-cells may explain the rise of autoimmune and atopic diseases observed in Western societies. SCFA-producing beneficial gut bacteria F. prausnitzii and Bifidobacterium are reduced in atopic dermatitis patients. The reduction is proportional to the severity of the presentation. E. coli has been associated with an increased risk of developing eczema, while C. difficile has been associated with an increased risk of atopic disease. The disrupted gut barrier and reduced SCFA levels can contribute to epithelial inflammation and the penetration of allergens and toxins, triggering immune responses associated with atopic dermatitis and leading to tissue damage in the skin.[20]
Psoriasis
The gut microbiome can influence the balance between immune tolerance and inflammation by modulating the differentiation of naive T-cells. The presence of beneficial gut species, such as A. muciniphila, Bacteroides, Proteobacteria, and F. prausnitzii, is reduced in psoriasis patients compared to healthy individuals. F. prausnitzii is important for producing butyrate, which provides energy for the cells lining the colon, reduces oxidative stress, and has anti-inflammatory effects by triggering regulatory T-cells. They also have higher levels of Actinobacteria and Firmicutes. The effects of intestinal dysbiosis in psoriasis may extend beyond the gastrointestinal system, as gut microbes and their metabolites can breach an impaired intestinal barrier and affect distant organs such as the skin and joints. DNA of gut microbial origin has been found in the blood of patients with active psoriasis.[20]
Ultraviolet (UV) damage and skin ageing
Some strains of the Lactobacillus species, when taken orally, exhibit anti-inflammatory properties and can delay the effects of UV-induced skin ageing. Mice that received Lactobacillus johnsonii supplements were protected from UV-induced hypersensitive reactions. This was attributable to decreased Langerhans cell counts in the skin and elevated interleukin-10 levels in the body. Supplementation with L. johnsonii La1 preserved a healthy immunological response in the skin after exposure to UV radiation by controlling the production of CD1a, a protein involved in interacting with T-cells.[20] Oral supplementation with L. plantarum resulted in improved skin elasticity, increased hydration, and inhibition of a protein called matrix metalloproteinase-1 expression in dermal fibroblasts, which is associated with skin ageing.[20]
Rosacea, hydradenitis suppurativa, and chronic spontaneous urticaria
Gut dysbiosis has been discovered in a group of Korean female rosacea patients. A study found that treating SIBO with rifaximin improved skin symptoms in rosacea patients.[11] Individuals with hydradenitis suppurativa have greater amounts of Ruminococcus gnavus and Clostridium ramosum than healthy controls.[19] Patients with chronic spontaneous urticaria have higher levels of Lactobacillus, Turicibacter, and Lachnobacterium and lower levels of Phascolarctobacterium.[3]
Overall, these findings highlight the complex interactions between the gut microbiome, gut barrier integrity, systemic inflammation, and various skin conditions. The gut-skin axis is an area of growing research, and understanding these connections may provide insights into new approaches for managing skin disorders. The reported alterations in gut microbiota in various skin diseases warrant more research and potential therapeutic approaches. “Will it be possible to build ourselves a better gut microbiota to promote healthy skin?” is a question that researchers ponder on.
FUTURE TREATMENT OPTIONS
Over recent years, gut microbiome-targeting therapies are gaining importance. FMT showed promising results for recurrent C. difficile infection in a study conducted in the Netherlands. Similarly, FMT might also be useful in the treatment of rosacea and atopic dermatitis.[22] Supplementation with probiotics and prebiotics has shown positive results in numerous skin disroders [Table 2].[23-31] In addition, developing vaccines against specific gut bacteria will work as a preventive method.[22] While the potential for microbiome-targeted therapies is exciting, one must remain cautious regarding the myths and misconceptions that surround this field as highlighted in an article in nature.[32]
| Probiotic strain | Disease treated | Authors |
|---|---|---|
| L. rhamnosus, B. longum, and Lactobacillus paracasei | Atopic dermatitis | Navarro-Lopez et al. Climent et al. |
| Lactobacillus plantarum, L. rhamnosus, Lactobacillus bulgaricus, and Streptococcus thermophiles | Acne, rosacea | Jung et al. Fabbrocini et al. Kim et al. |
| B. longum, Bifidobacterium infantis, Bifidobacterium lactis, and L. rhamnosus | Psoriasis | Navarro-Lopez et al. Groeger et al. |
| Lactobacillus kunkei | Alopecia areata | Rinaldi et al. |
| Lactobacillus fermentum, Lactobacillus reuteri, and Bacillus subtilis | Wounds | Golkar et al. |
L. rhamnosus: Lactobacillus rhamnosus, B. longum: Bifidobacterium longum
CONCLUSION
The gut microbiome is essential in maintaining the balance between health and disease. Unfortunately, current lifestyles marked by high levels of stress, fewer social interactions, less outdoor exposure, a sterile environment, diets deficient in fiber, and excess antibiotic use can upset this equilibrium and contribute to the development of numerous disorders.[7] “Let food be thy medicine, and medicine be thy food” dates back to Hippocrates, the father of medicine. This statement is still pretty relevant today and stresses the importance of a healthy diet to maintain good health. The food we consume can take up both preventive and therapeutic roles. Diet and microbiome interactions and their impact on the host organism have received international interest and are quickly evolving. Over the past two decades, gut microbiota has gained lots of attention, with new updates almost every day. As the field advances, so does the proliferation of exaggerated claims. Nature recently published an article highlighting the myths and misconceptions regarding the human microbiome.[32] One prevalent misconception suggests that the gut microbiome’s functionality is redundant. While some vital functions, like producing SCFAs, are widespread, certain roles, such as degrading oxalate and resistant starch, are unique to specific microbiota species. Other members of the microbiota cannot replace these specific functions. Another fallacy centers on the Firmicutes: Bacteroidetes ratio and its supposed connection to obesity. Despite animal studies hinting at its relevance, several meta-analyses have contradicted these claims in human studies. Discrepancies between these studies underscore the absence of reliable microbial taxonomic indicators for obesity. This necessitates a cautious approach to resource allocation and highlights the potential risk of undermining public trust due to such inconsistencies. Addressing these misconceptions is vital before dedicating finite resources to further research grounded in unverified claims. Trials are being conducted to find the “magic bullet,” that is, which microbe or microbial communities are required to prevent or treat a particular disease. We believe we might not be too far away from a world where microbes can be used not just to diagnose diseases but also to treat them quite efficiently as well.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent was not required as there are no patients in this study.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- The healthy microbiome-what is the definition of a healthy gut microbiome? Gastroenterology. 2020;160:483-94.
- [CrossRef] [PubMed] [Google Scholar]
- Missing microbes: How the overuse of antibiotics is fueling our modern plagues New York: Henry Holt and Company; 2014.
- [Google Scholar]
- Human microbiome myths and misconceptions. Nat Microbiol. 2023;8:1392-6.
- [CrossRef] [PubMed] [Google Scholar]
- Ted books box set: The science mind: Follow your gut, how we'll live on mars, and the laws of medicine United States: Simon and Schuster/TED; 2015.
- [Google Scholar]
- Gut microbiome In: Adult short bowel syndrome. Cambridge: Academic Press; 2019. p. :45-54.
- [CrossRef] [Google Scholar]
- Correlations of host genetics and gut microbiome composition. Front Microbiol. 2016;7:1357.
- [CrossRef] [PubMed] [Google Scholar]
- Gut microbiome composition and diversity are related to human personality traits. Hum Microbiome J. 2020;15:100069.
- [CrossRef] [PubMed] [Google Scholar]
- Human gut microbiota/microbiome in health and diseases: A review. Antonie Van Leeuwenhoek. 2020;113:2019-40.
- [CrossRef] [PubMed] [Google Scholar]
- The origins of gut microbiome research in Europe: From Escherich to Nissle. Hum Microbiome J. 2019;14:100065.
- [CrossRef] [Google Scholar]
- From hydrotherapy to the discovery of the gut microbiota: The historical gastrointestinal health concept. Pharmacophore. 2020;11:82-90.
- [Google Scholar]
- The gut microbiome: Human health and inflammatory skin diseases. Ann Dermatol. 2020;32:265-72.
- [CrossRef] [Google Scholar]
- Communication between the gut microbiota and peripheral nervous system in health and chronic disease. Gut Microbes. 2022;14:2068365.
- [CrossRef] [PubMed] [Google Scholar]
- Gut-on-a-chip: Current progress and future opportunities. Biomaterials. 2020;255:120196.
- [CrossRef] [PubMed] [Google Scholar]
- Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes. 2016;7:189-200.
- [CrossRef] [PubMed] [Google Scholar]
- Regulation of human epithelial tight junction proteins by Lactobacillus plantarum in vivo and protective effects on the epithelial barrier. Am J Physiol Gastrointest Liver Physiol. 2010;298:G851-9.
- [CrossRef] [PubMed] [Google Scholar]
- The gut-immune connection: How understanding the connection between food and immunity can help us regain our health New York: Ru Guo; 2022.
- [Google Scholar]
- Microbiome in the gut-skin axis in atopic dermatitis. Allergy Asthma Immunol Res. 2018;10:354-62.
- [CrossRef] [PubMed] [Google Scholar]
- Establishing what constitutes a healthy human gut microbiome: State of the science, regulatory considerations, and future directions. J Nutr. 2019;149:1882-95.
- [CrossRef] [PubMed] [Google Scholar]
- Altered skin and gut microbiome in hidradenitis suppurativa. J Invest Dermatol. 2022;142:459-68.e15.
- [CrossRef] [PubMed] [Google Scholar]
- The gut microbiome as a major regulator of the gut-skin axis. Front Microbiol. 2018;9:1459.
- [CrossRef] [PubMed] [Google Scholar]
- The human microbiome: History and future. J Pharm Pharm Sci. 2020;23:404-11.
- [CrossRef] [PubMed] [Google Scholar]
- Abnormalities in gut microbiota and metabolism in patients with chronic spontaneous urticaria. Front Immunol. 2021;12:691304.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of oral administration of a mixture of probiotic strains on SCORAD index and use of topical steroids in young patients with moderate atopic dermatitis: A randomized clinical trial. JAMA Dermatol. 2018;154:37-43.
- [CrossRef] [PubMed] [Google Scholar]
- Changes in gut microbiota correlates with response to treatment with probiotics in patients with atopic dermatitis. A post hoc analysis of a clinical trial. Microorganisms. 2021;9:854.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy and safety of oral administration of a mixture of probiotic strains in patients with psoriasis: A randomized controlled clinical trial. Acta Derm Venereol. 2019;99:1078-84.
- [CrossRef] [PubMed] [Google Scholar]
- Bifidobacterium infantis 35624 modulates host inflammatory processes beyond the gut. Gut Microbes. 2013;4:325-39.
- [CrossRef] [PubMed] [Google Scholar]
- Prospective, randomized, open-label trial comparing the safety, efficacy, and tolerability of an acne treatment regimen with and without a probiotic supplement and minocycline in subjects with mild to moderate acne. J Cutan Med Surg. 2013;17:114-22.
- [CrossRef] [PubMed] [Google Scholar]
- Dietary effect of lactoferrin-enriched fermented milk on skin surface lipid and clinical improvement of acne vulgaris. Nutrition. 2010;26:902-9.
- [CrossRef] [PubMed] [Google Scholar]
- Supplementation with Lactobacillus rhamnosus SP1 normalises skin expression of genes implicated in insulin signalling and improves adult acne. Benef Microbes. 2016;7:625-30.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy of postbiotics in a PRP-like cosmetic product for the treatment of alopecia area celsi: A randomized double-blinded parallel-group study. Dermatol Ther. 2020;10:483-93.
- [CrossRef] [PubMed] [Google Scholar]
- A novel effective formulation of bioactive compounds for wound healing: Preparation, in vivo characterization, and comparison of various postbiotics cold creams in a rat model. Evid Based Complement Altern Med. 2021;2021:8577116.
- [CrossRef] [PubMed] [Google Scholar]
- Dysregulation of the gut-brain-skin axis and key overlapping inflammatory and immune mechanisms of psoriasis and depression. Biomed Pharmacother. 2021;137:111065.
- [CrossRef] [PubMed] [Google Scholar]






