Translate this page into:
Confocal microscopy – Working principle and applications in dermatology
*Corresponding author: Rakhe Jayamohanan, Department of Dermatology, Mampilly Medical Center, Sharjah, United Arab Emirates. rakhejayamohanan@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Jayamohanan R. Confocal microscopy – Working principle and applications in dermatology. J Skin Sex Transm Dis 2023;5:81-9.
Abstract
Confocal microscopy (CFM) is a novel, non-invasive tool with diagnostic and prognostic value. It has utility in a wide array of dermatological conditions, tele-dermatopathology, and dermatological research. The image contrast is imparted by the differential refractive properties of subcellular structures. Certain stains are also used to accentuate the image contrast. CFM enables the visualization of tissues both in vivo and ex vivo (after excision). Both reflectance (in vivo and ex vivo) and fluorescence modes (ex vivo) of CFM are utilized for imaging. One can view 3D images with a near histological resolution, but with a limited depth of penetration. CFM has reduced the number of biopsies required in the evaluation of skin cancers. However, conventional histopathology remains the gold standard in tumor diagnosis. The in vivo reflectance mode CFM has found applications in dermato-oncology, inflammatory dermatoses, cutaneous infections and infestations, skin aging, and pigmentary disorders. The ex vivo CFM facilitates the immediate perioperative examination of excised tissues. The limited depth of view, photobleaching (in fluorescence mode CFM), high cost, and steep learning curve are the disadvantages. The future of CFM appears promising with the introduction of modified microscopes, the use of specific stains, and the incorporation of artificial intelligence.
Keywords
Confocal microscopy
Reflectance confocal microscopy
Fluorescence confocal microscopy
Non-invasive 3D imaging
INTRODUCTION
Over the past three decades, confocal microscopy (CFM) has evolved as a useful, non-invasive, imaging technology that has diagnostic and prognostic implications.[1] Modifications in technology have encouraged its use in a wide spectrum of skin conditions such as melanomas, skin tumors, melanocytic lesions, inflammatory dermatoses, and infections.[2,3] CFM has found applications in dermatology research and tele-dermatopathology.[4] This article delves into the nuance of the working principles of CFM, its major differences from conventional microscopy, and the uses, advantages, and disadvantages of the technique.
HISTORY
Marvin Minsky first described the basic principles of confocal laser scanning microscopy in 1955 and patented the technology.[1] However, it was not until the 1990s, following the development of reflectance mode confocal laser scanning microscopy by Rajadhayaksha et al. that this technology was further explored.[1] With innovations in optics and improvements in the design of the microscope, various modifications have been introduced. Nowadays, handheld devices are also available.[5]
WORKING PRINCIPLE AND OPTICS OF CONFOCAL MICROSCOPE
The technique of CFM emerged as an ideal method to overcome the two limitations of a fluorescence microscope. A fluorescence microscope is a widefield microscope that uses a mercury arc lamp as the light source that emits white light of various wavelengths [Figure 1]. Excitation filter in the pathway of light, filters out all wavelengths, except those needed to excite the fluorochrome. It uses a dichroic mirror, a specialized mirror calibrated to reflect these excitation wavelengths toward the specimen, but transmits longer emission wavelengths. The objective lens, then focuses the excitation wavelength to the specimen, producing a fluorescence that has a wavelength longer than the excitation light. These emission wavelengths pass through the emission filter, which permits only the passage of wavelengths above and below a specific range. These emission wavelengths then reach the detector, which assists in the detection, visualization, and measurement of the fluorescent signals, forming an image.[6] In a fluorescence microscope, the whole specimen is illuminated with a white light source. The reflected light in its entirety is viewed as an image by the observer. Hence, fluorescence from a large area of the observed field is obtained, which, in turn, gives a lot of background “noise” of fluorescence, compromising the image resolution.[6] An added limitation is the time needed for preparing the specimen before staining and microscopy.[6]

- Schematic diagram of an epifluorescence microscope.
A confocal microscope [Figure 2] is a modified fluorescence microscope.[7] It uses lasers as a light source, replacing the white light with a specific wavelength, thus eliminating the need for excitation and emission filters. Lasers act as a point source of light, restricting the illumination to a tiny spot within the field of view. This gives a concise and defined illumination volume in contrast to widefield microscopy, where the white light unrestrictedly illuminates everything within the field of view. The second modification is the placement of pinholes at two critical focal points (the conjugate focal points – hence, the term “confocal” microscope) in the pathway of light. The first one is placed near the light source and the second one near the detector. These pinholes allow only the rays from the area under focus to pass through them and cut off all the rest. This creates an image with superior resolution (since much of the background fluorescence is eliminated).[1,6,7] Since the laser light is a point source, only a speck of the specimen is focused at any given time. Hence, to obtain the image of the specimen in its entirety, the laser light is run horizontally from one end of the field to the other – a process called scanning – producing an image mosaic of the specimen. Now, by running similar scans at planes above and below these focal planes, multiple 2D images can be obtained at different depths of the specimen, which are then combined using a computer program to construct a 3D image.[7]
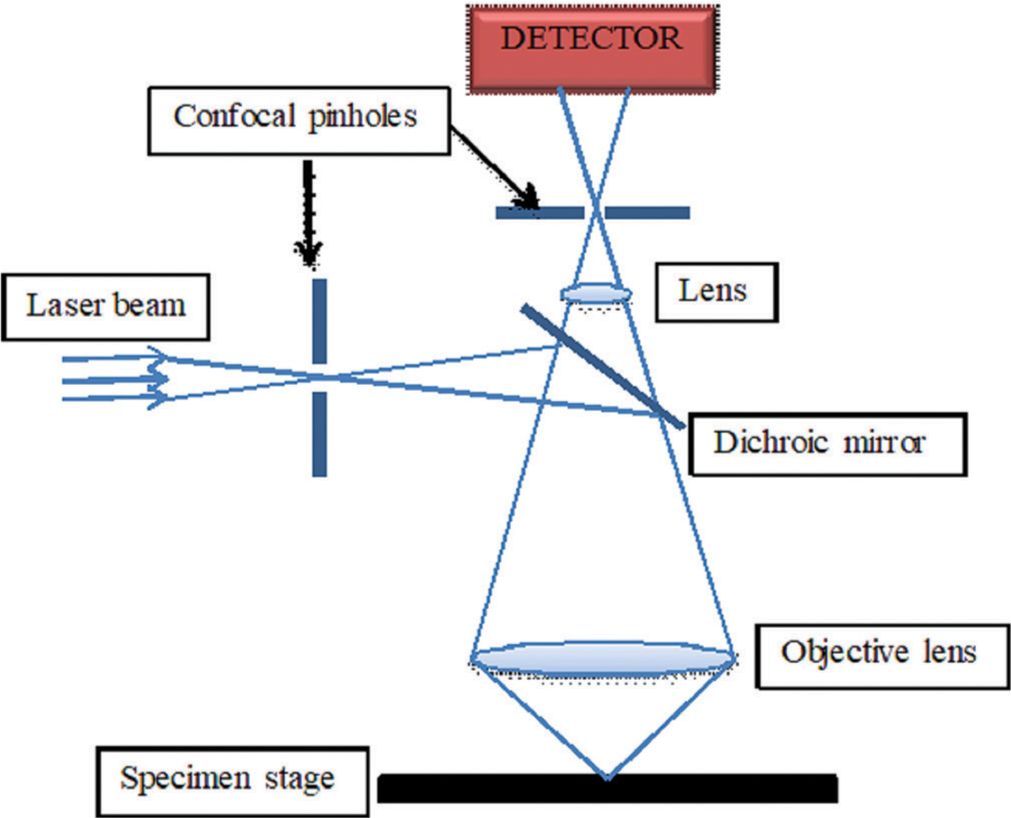
- Schematic diagram of a confocal microscope.
IMAGING WITH CONFOCAL MICROSCOPE
The CFM can be used to examine both living (in vivo CFM) as well as excised tissues (ex vivo CFM).[5,8]
In vivo CFM
In vivo CFM [Figure 3] predominantly works in reflectance mode (reflectance confocal microscope, RCM). The image contrast is provided by the differential refractive properties of the subcellular structures. No special staining is required in reflectance mode, though contrast agents such as aluminum chloride, citric acid, or acetic acid can accentuate the nuclei.[9,10] Keratin, melanin, and collagen have greater refractive indices than water and act as endogenous chromophores in the skin.

-
In vivo confocal laser scanning microscope (830 nm), the wide-probe confocal device (VivaScope®1500, Image courtesy – VivaScope GmbH, Munich, Germany).
They appear brighter, and hence whiter, compared to the rest of the structures which are darker and hence appear black/ grey.[2] This produces an image contrast in grayscale.[2] In vivo RCM is used to examine directly accessible tissues such as skin, mucosa, and eyes and endoscopically viewed structures such as the gastrointestinal tract and genitourinary tract.[11-13] There are two different models of RCM for in vivo examination of skin – a large device called a wide-probe confocal device having one articulated arm and a handheld device.[4] Both models use diode laser at 830nm wavelength.[1,5] With in vivo CFM, one can explore the living tissue at a near histological resolution, visualizing the epidermis, dermo-epidermal junction, and upper dermis up to a depth of 200–250 µm.[13-15]
Normal skin under in vivo RCM
The appearance of the normal skin under in vivo RCM has been described.[5,14] Shahriari et al. elaborately defined terminologies used in RCM and elucidated the same with detailed images.[15] As the RCM images are generated from top to bottom, in normal skin, the first layer encountered is the stratum corneum. The keratinocytes are polygonal cells with decreasing size from stratum corneum to stratum basale.[5,15] The stratum corneum [Figure 4a] appears bright and characterized by flat, polygonal, and anucleate cells.[5] The flattened granular keratinocytes have dark central nuclei with a surrounding rim of refractile and granular cytoplasm [Figure 4b].[5] The granular layer manifests a honeycomb pattern. The pattern is imparted by the polygonal, bright cellular outlines of keratinocytes that are arranged back to back.[5,15] The honeycomb pattern is seen in stratum spinosum [Figure 4c] also.[5] The keratinocytes of the basal layer [Figure 4d] are brighter at the center in comparison to the peripheral cytoplasm.[5] This is attributed to the melanin caps that enclose the nuclei.[5] The bright, round basal keratinocytes above dermal papillae give a cobblestone pattern.[5] The characteristic feature of the dermo-epidermal junction is referred to as “edged papilla.”[5] The appearance is produced by rings of basal keratinocytes that surround dark circular structures (dermal papillae) with collagen and blood capillaries within it. The melanocytes, pigmented keratinocytes, and Langerhans cells (when metabolically active in the inflammatory phase) strongly reflect the 830 nm laser light and appear bright.[5] However, in normal human skin, it is difficult to distinguish between melanocytes and pigmented keratinocytes. Furthermore, in their quiescent state, it is difficult to visualize Langerhans cells clearly.15]

- (a): In vivo reflectance confocal microscopy image of stratum corneum – corneocytes appear as large, polygonal cells without a visible nucleus. Islands of keratinocytes are separated by linear, dark fissures that represent dermatoglyphics (Image courtesy – VivaScope GmbH, Munich, Germany); (b): In vivo reflectance confocal microscopy image of stratum granulosum showing keratinocytes (central dark nuclei and surrounding refractile granular cytoplasm) with well-demarcated outlines giving a honeycomb pattern (Image courtesy – VivaScope GmbH, Munich, Germany); (c): In vivo reflectance confocal microscopy image of stratum spinosum showing smaller, polygonal cells with thin, white cytoplasm and oval, dark nuclei. Cells of stratum spinosum also form a honeycomb pattern (Image courtesy – VivaScope GmbH, Munich, Germany); (d): In vivo reflectance confocal microscopy image of stratum basale showing small basal cells with uniform size and shape. The cells in this layer appear brighter in the center due to the presence of melanin caps encasing the nuclei (Image courtesy – VivaScope GmbH, Munich, Germany).
Collagen fibers are seen in a reticulated pattern in the superficial dermis, while they appear as thick bundles arranged parallelly in the deep, reticular dermis.[5]
Ex vivo CFM
Ex vivo CFM has both reflectance and fluorescence modes.[10,13,14] The former uses a laser wavelength of 830 nm and the latter uses a laser wavelength of 488 nm or 658 nm.[13,14] The fluorescence mode confocal microscope (FCM) uses exogenous fluorochromes to enhance the image contrast. Acridine orange is the most widely used agent, which fluorescently stains the nuclei of cells white.[8,13,14] The other fluorochromes used are methylene blue, toluidine blue, Nile blue, fluorescein, and patent blue V.[8,13,14] Acridine orange does not alter the tissue specimens. Therefore, these specimens can be taken up for a further frozen section or conventional histopathology.[13]
In ex vivo RCM, the specimen is placed en masse between two thick glass slides, which are glued together with silicone glue or modeling clay. To ensure adequate contact between the specimen and the glass slide, the viewing side is smeared with aqueous gel or silicone. Furthermore, the specimen is flattened using a tissue press for better contact. This can then be placed on the specimen stage for viewing in RCM/FCM modes.[13] The ex vivo CFM can image up to a depth of 200 µm corresponding to the upper dermis, producing horizontal images of 750 × 750 µm, which are combined to produce an image mosaic of 20 × 20 mm.[10,14]
The latest generation FCM employs two wavelengths of laser (488 nm blue and 758 nm infrared).[16] In fluorescent mode, dyes such as acridine orange are excited by the blue laser and the nuclear structures stained by them appear green, while the infrared laser highlights melanin and keratin in reflectance mode, which appear in different shades of grey.[16]
One limitation encountered in CFM in comparison to routine histopathology was that fluorescence mode showed only nuclear detail and did not show collagen or cytoplasm.[17] This was comparable to the role of hematoxylin in histopathology. The contrast provided by reflectance mode (without staining) was sensitive only to collagen and cytoplasm, which was comparable to eosin in histopathology.[17] Gareau described “digitally stained confocal mosaics (DSCMs) that combined confocal fluorescence and reflectance images in a multimodal pseudo-color image which simulated staining with hematoxylin and eosin” and improved the sensitivity and specificity of CFM.[16,17] Mu et al. reported that DSCMs improved the diagnostic accuracy of physicians (with training) to detect non-melanoma skin cancers.[18]
DIFFERENCES BETWEEN CFM AND CONVENTIONAL FLUORESCENCE MICROSCOPY
The differences between the CFM and conventional fluorescence microscope lie in three key areas [Table 1].[1,6,7] First, the RCM mode utilizes 830 nm and FCM mode 488 nm and 658 nm wavelength lasers.[1] This means that the light source used in CFM is a point source of laser light. Being monochromatic, the desired wavelength can be attained without a filter.[6,7] Second, there are strategically placed pinholes at conjugate focal planes in CFM, one near the light source and the other near the detector. These pinholes assist in increasing the resolution by removing all the out of focus rays above and below the focal plane.[7] Third, since only a small spec of tissue is visualized at a time, scanning mode is utilized to get the entire image from the focal plane in the form of a mosaic and the final image is of superior resolution, when compared to epifluorescence microscope.[7] Needless to say, the time required to obtain an image using the confocal technique is much less as tissue processing or staining is not a prerequisite for tissue imaging.
| Parameters | Confocal microscope | Conventional fluorescence microscope |
|---|---|---|
| Light source | Monochromatic laser | Mercury arc lamp-white light |
| Pinholes | Two pinholes – one each at conjugate focal planes | Absent |
| Filters | Not required | Emission and excitation filters required |
| Scanning | The specimen is scanned to create an image mosaic | Widefield microscopy – whole specimen is illuminated |
| Resolution | Superior – as focused and point source of light illuminates a tiny bit of tissue at a time, eliminating background illumination | Blurring effect due to background illumination |
| In vivo/ex vivoutility | Confocal microscope can be used to examine both live (reflectance confocal microscopy) and excised tissues (reflectance confocal microscopy and fluorescence mode confocal microscopy) | Tissues have to be excised, processed, fixed, and stained before viewing |
| Time needed | Few minutes to hours | Hours to days |
APPLICATIONS OF CFM IN DERMATOLOGY
The CFM has found a myriad of applications in both the clinical and research fields of dermatology. In vivo RCM has found utility in dermato-oncology, inflammatory dermatoses, cutaneous infections and infestations, skin aging, and pigmentary disorders to name a few.[2,3] Basal cell carcinoma (BCC) is the most widely studied entity with ex vivo CFM and commonly compared with conventional histopathology. It has also been used to study other tumors and dermatoses.[3,5]
In vivo CFM
Melanoma
The major features for a diagnosis of melanoma include identification of atypical cells in the epidermis, architectural disorder at the dermo-epidermal junction (DEJ disarray), and atypical/irregular aggregate of melanocytes in the dermis. Pellacani et al. proposed a diagnostic algorithm for malignant melanoma in 2005.[19] A subsequent study identified “epidermal disarray and pagetoid cells in the epidermis, non-edged papillae, and cellular atypia at the junction, and atypical nests and bright nucleated cells in the upper dermis” as RCM features indicating melanomas. The authors noted “regular dermoepidermal architecture, and absence of pagetoid infiltration and atypical cells” as features favoring benign lesions.[20]
Segura et al. described a two-step method for the diagnosis of melanoma. In the first step, melanocytic lesions were differentiated from non-melanocytic lesions. In the second step, melanoma was distinguished from melanocytic nevi.[21]
A systematic review found a sensitivity of 93% and specificity of 76% for the diagnosis of melanoma using RCM.[22] Pellacani et al., in a randomized clinical trial on suspect lesions concluded that “adjunctive use of RCM reduced unnecessary excisions and assured the removal of aggressive melanomas at baseline in a real life. All lesions, where a diagnosis of melanoma was delayed, were thinner than 0.5 mm.”[23]
Castro et al., after studying 110 lesions that were clinically and dermoscopically suspicious of superficial spreading melanoma, reported three RCM features that showed statistically significant association with melanomas and they included “peripheral hotspot at the dermo-epidermal junction (defined as 1 mm × 1 mm area of the lesion where atypical cells are more aggregated),” “nucleated roundish cells at the dermo-epidermal junction,” and “sheet of cells.”[24]
Handheld RCM is used to determine the tumor extent before definitive surgery. There was an overall 85.9% agreement between margin assessment by RCM and final histopathology.[25]
BCC
The sensitivity and specificity of RCM for diagnosis of BCC, reported in a meta-analysis, were 97% and 93%, respectively.[26] The features pointing to a diagnosis of BCC on RCM are noted in the superficial dermis or at the dermo-epidermal junction. The characteristic features that indicate BCC are “dark silhouettes (hyporeflective zones surrounded by bright collagen bundles), bright tumor islands which are often limited by a dark cleft, dendritic cells, plump, bright cells, and canalicular vessels.” The main distinguishing feature of superficial BCC is epithelial cords which are attached to the epidermis. Nodular BCC shows the additional feature of increased vascular density. Hyporeflective areas mark aggressive subtypes.[27]
Bowen’s disease/in situ squamous cell carcinoma (SCC)
Atypical honeycomb pattern, disruption of stratum corneum, S-shaped blood vessels, and targetoid cells are observed on RCM. However, it shows lesser degree of keratinocyte atypia and architectural distortion, when compared to SCC.[13]
Invasive SCC
Ulcerations in SCC correspond to dark areas with sharp irregular contours filled with amorphous material. The characteristic features on CFM are architectural disarrangement, round/polygonal cells with speckled appearance, dark nuclei in the epidermis, and irregular, dilated blood vessels.[13]
Actinic keratosis (AK)
On RCM, AK shows disarranged/mildly atypical honeycomb pattern, nuclear retention (parakeratosis), and bundles of collagen in the superficial dermis.[13]
Other applications of in vivo RCM
Inflammatory skin conditions
RCM features of psoriasis, lichen planus, and discoid lupus erythematosus showed correlation with histology [Table 2].[28-31] In vivo RCM was also found useful in differentiating scarring alopecia from its non-scarring counterpart.[32,33]
| Psoriasis | Thickened stratum corneum (hyperkeratosis) with small dark nuclei within the bright cells of stratum corneum (parakeratosis) Clusters of refractile, round/polygonal cells between corneocytes (Munro’s microabscess), reduced or absent granular layer (hypogranulosis), and thickened stratum spinosum (acanthosis) Increase in the diameter and density of dermal papillae (papillomatosis) in horizontal RCM section, dilated blood vessels surrounded by refractile inflammatory infiltrate in superficial dermis |
| Lichen planus | Increased intercellular spaces (spongiosis), large, polygonal cells (hypergranulosis), thickened wedge shaped granular layer (Wickham’s striae), grouped round/polygonal bright cells (inflammatory infiltrate) Smeared refractile rings replace the normally bright refractile structures around dermal papillae at DEJ (vacuolar degeneration of basal layer), superficial dermis shows bright, plump, large, oval/stellate cells (melanophages), and dilated blood vessels |
| Discoid lupus erythematosus | Roundish areas filled with highly refractive, amorphous material (dilated follicles and follicular plugging) at the level of stratum corneum, atypical honeycomb pattern in epidermis (structural keratinocyte disarrangement), small, polygonal/round, bright cells (inflammatory cells), DEJ showing mild disarrangement and several polygonal/round, bright cells, and some dendritic cells (partial or total disappearance of dermal papillae attributed to interface dermatitis) |
RCM: Reflectance confocal microscopy, DEJ: dermoepidermal junction
Cutaneous wound healing
This is an important research field, where in vivo RCM could visualize the inflammatory, vascular, and tissue remodeling features of wound healing in real time. Furthermore, in vivo RCM can predict the course of wound healing in burn injury of indeterminate depth by serially examining the microcirculation, morphology, and inflammatory cell traffic.[28]
Skin aging
In vivo RCM examination shows keratinocyte alterations, irregular pigmentation, and increased compactness of collagen fibers with aging. Effect of ultraviolet radiation is visualized as skin inflammation, microvesicle formation, apoptotic keratinocytes, activated melanocytes, and loss of epidermal structure in a sequential order.[28]
Research
In vivo RCM is used for the investigation of components of skin inflammation, namely, leukocyte migration and capillary blood flow.[28] It can be an imaging tool in the study of skin reactivity to topical stimuli and interpretation of equivocal patch test. In a study, 40% of equivocal patch test reactions showed positive allergic reaction pattern on in vivo RCM imaging.[28]
Applications of ex vivo CFM
The utility of ex vivo CFM is in the immediate perioperative examination of an excised tissue in its entirety, thus bypassing the need for tissue processing. Bennàssar et al. proposed FCM criteria for diagnosis of BCC (peripheral palisading, clefting, nuclear pleomorphism, and presence of stroma).[9] Stroma refers to the modified dermis that surrounds the tumor mass. In FCM, it gives the characteristic “starry sky” appearance.[9] “Inflammatory cells and activated fibroblast nuclei” correspond to the “stars.”[9] Authors identified certain features as characteristics of specific subtypes [Table 3].[9]
| BCC subtype | Fluorescent confocal microscopy features |
| Superficial BCC | Sparsely distributed fluorescent dots (tumoral nuclei) along the basal layer of the epidermis, absence of crowding images, predominance of ill-defined lesions, presence of clefting, and less stromal reaction, and inflammation in comparison to nodular and infiltrative types. |
| Nodular BCC | Well-demarcated lesions, nodular crowding of hyperfluorescent, pleomorphic nuclei with high nuclear/cytoplasmic ratio, fluorescent dots representing tumoral nuclei arranged parallel in periphery, stroma showing the typical “starry sky” pattern, and black fluorescence-free areas (clefting) separating tumoral dermis from the main islands of BCC. |
| Infiltrating BCC | Fluorescent pleomorphic dots forming nests and strands which infiltrate the surrounding dermis, strong stromal and inflammatory reaction, and thinner strands appearing as more ill-defined lesions and showing less nuclear crowding, palisading, and clefting. |
BCC: Basal cell carcinoma
Ruini et al., in a study on “frozen sections of BCCs, stained with acridine orange and digitally colored to simulate hematoxylin and eosin dyes,” found FCM to have a sensitivity and specificity of 92% and 91%, respectively, to identify tumor-free margins (in comparison to conventional histopathology).[16] A concordance of 88% was noted between the two techniques in the subtype classification of BCC.[16]
Besides its use in assessing tumor margins in BCC, ex vivo CFM has various other indications including identification and grading of the degree of differentiation/invasiveness of SCC, diagnosis of melanoma, and measurement of tumor thickness in melanoma.[10] This may help to determine the disease-free tumor margins during excision.
Ex vivo RCM helps to confirm nail tumors intraoperatively, which, in turn, makes immediate excision possible without tissue wastage.[10]
ADVANTAGES OF CFM
High-resolution, high-contrast images of the entire epidermis and upper dermis are obtained with a near histological view of the tissues with good histological correlation.[10] These can be reconstructed into 3D images. The absence of artifacts as seen in conventional microscopy such as shrinkage, loss of fat, and absent blood flow is an added advantage. The time required for the study is much less compared to conventional histopathology.[34]
Limitations
The greatest disadvantage of CFM is the shallow depth of imaging due to the limited depth of optical penetration. As a result, the mid and deep dermis cannot be visualized.[34] Hence, the depth of the lesion cannot be assessed and in doubtful cases, lesions are better biopsied and subjected to histopathology evaluation to rule out an invasive process. Unlike conventional histology where vertical sections are the norm, here, horizontal sections are analyzed.[14] CFM has a steep learning curve in image interpretation for practitioners in clinical practice.[35] Another major concern of CFM is the high cost compared to conventional microscopy/dermoscopy.
RCM has increased the diagnostic accuracy in skin cancers, thus reducing the number of invasive biopsies.[13] However, histopathology still remains the gold standard in the diagnosis of skin cancers. RCM produces grayscale images which may be difficult to interpret unlike conventional hematoxylin and eosin stained specimens, where good image contrast is obtained.[13] RCM produces poor quality pictures on the acral skin due to increased thickness of stratum corneum.[31]
Photobleaching (chemical alterations on exposure to light that result in a molecule losing its ability to fluoresce) of fluorescent probes and phototoxicity of live samples – the limitations of conventional fluorescence microscopy – are the limitations of FCM also.
FUTURE PROSPECTS
Recent studies with newer and faster microscopes using fusion images from RCM and FCM modes render images that are similar to hematoxylin and eosin stained specimens.[17,18] Gareau et al. used a new line of scanning (the line scanning, stage scanning confocal microscope, LSSSCM), which used 488 nm and 532 nm laser lights to examine BCC; acridine orange was the fluorescent stain.[36] The LSSSCM has no scanning mirrors and performs faster scan with cellular resolution.[36]
The in vivo RCM can be used by a trained technician/clinician to acquire images at the clinician’s office which can then be sent to an expert diagnostic reader. This way, a telepathology network may be set up to receive a diagnosis in <30 minutes. This offers great potential to assess tumor response to topical therapies. Over time, this can also be employed to delineate tumor margins before surgery (pre-surgical mapping).[4]
CONCLUSION
The confocal microscope has come a long way since its inception. There is renewed interest in this noninvasive imaging modality as there is a preference for less invasive diagnostic tools in dermatology. In this light, the CFM holds a promising future, despite its limitations including the steep learning curve for the practitioner and the cost of the equipment. It is now an emerging tool in the diagnostic armamentarium for dermatologists/ dermatopathologists that can complement existing technologies like dermoscopy. The scope of CFM in telepathology was recently reviewed with the practitioner performing the image capture and sending it to a trained dermatopathologist for viewing. This considerably reduces the patient’s discomfort, and the time needed to obtain a diagnosis and plan a treatment strategy.[4] The technical advance in CFM has been considerable. The development of software tools for image processing and analysis, and the incorporation of artificial intelligence and machine learning algorithms are the way forward for CFM.[7]
Declaration of patient consent
Not required as patient’s identity is not disclosed or compromised.
Conflicts of interest
There are no conflicts of interest.
Financial support and sponsorship
Nil.
References
- History and fundamentals of reflectance confocal microscopy In: Fimiani M, Rubegni P, Cinotti E, eds. Technology in Practical Dermatology. Switzerland: Springer Nature; 2020. p. :127-34.
- [CrossRef] [Google Scholar]
- In vivo confocal microscopy in dermatology: From research to clinical application. J Biomed Opt. 2013;18:61212.
- [CrossRef] [PubMed] [Google Scholar]
- Reflectance confocal microscopy for cutaneous infections and infestations. J Eur Acad Dermatol Venereol. 2016;30:754-63.
- [CrossRef] [PubMed] [Google Scholar]
- Reflectance confocal microscopy: An overview of technology and advances in telepathology. Cutis. 2015;95:E39-46.
- [Google Scholar]
- Reflectance confocal microscopy: Principles, basic terminology, clinical indications, limitations, and practical considerations. J Am Acad Dermatol. 2021;84:1-14.
- [CrossRef] [Google Scholar]
- Fluorescence microscopy. Cold Spring Harb Protoc. 2014;2014:pdb.top071795.
- [CrossRef] [PubMed] [Google Scholar]
- Confocal microscopy: Principles and modern practices. Curr Protoc Cytom. 2020;92:e68.
- [CrossRef] [PubMed] [Google Scholar]
- Ex vivo confocal microscopy: Revolution in fast pathology in dermatology. Br J Dermatol. 2020;183:1011-25.
- [CrossRef] [PubMed] [Google Scholar]
- Fast evaluation of 69 basal cell carcinomas with ex vivo fluorescence confocal microscopy: Criteria description, histopathological correlation, and interobserver agreement. JAMA Dermatol. 2013;149:839-47.
- [CrossRef] [PubMed] [Google Scholar]
- Ex vivo confocal microscopy: An emerging technique in dermatology. Dermatol Pract Concept. 2018;8:109-19.
- [CrossRef] [PubMed] [Google Scholar]
- In vivo reflectance confocal microscopy in general dermatology: How to choose the right indication. Dermatol Pract Concept. 2020;10:e2020032.
- [CrossRef] [PubMed] [Google Scholar]
- Confocal laser endomicroscopy in the “in vivo” histological diagnosis of the gastrointestinal tract. World J Gastroenterol. 2009;15:5770-5.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical applications of in vivo and ex vivo confocal microscopy. Appl Sci. 2021;11:1979.
- [CrossRef] [Google Scholar]
- “Confocal Microscopy”, Dermoscopedia. Available from: https://dermoscopedia.org/w/index.php?title=Confocal_Microscopy&oldid=16675 [Last accessed on 2021 Jun 16]
- [Google Scholar]
- In vivo reflectance confocal microscopy image interpretation for the dermatopathologist. J Cutan Pathol. 2018;45:187-97.
- [CrossRef] [PubMed] [Google Scholar]
- Ex-vivo fluorescence confocal microscopy with digital staining for characterizing basal cell carcinoma on frozen sections: A comparison with histology. J Biophotonics. 2021;14:e202100094.
- [CrossRef] [PubMed] [Google Scholar]
- The feasibility of digitally stained multimodal confocal mosaics to simulate histopathology. J Biomed Opt. 2009;14:34050.
- [CrossRef] [PubMed] [Google Scholar]
- Use of digitally stained multimodal confocal mosaic images to screen for nonmelanoma skin cancer. JAMA Dermatol. 2016;152:1335-41.
- [CrossRef] [PubMed] [Google Scholar]
- Reflectance-mode confocal microscopy of pigmented skin lesions--improvement in melanoma diagnostic specificity. J Am Acad Dermatol. 2005;53:979-85.
- [CrossRef] [PubMed] [Google Scholar]
- The impact of in vivo reflectance confocal microscopy for the diagnostic accuracy of melanoma and equivocal melanocytic lesions. J Invest Dermatol. 2007;127:2759-65.
- [CrossRef] [PubMed] [Google Scholar]
- In vivo microscopic features of nodular melanomas: Dermoscopy, confocal microscopy, and histopathologic correlates. Arch Dermatol. 2008;144:1311-20.
- [CrossRef] [PubMed] [Google Scholar]
- Systematic review of diagnostic accuracy of reflectance confocal microscopy for melanoma diagnosis in patients with clinically equivocal skin lesions. Dermatol Pract Concept. 2013;3:19-27.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of reflectance confocal microscopy for suspect lesions on diagnostic accuracy in melanoma: A randomized clinical trial. JAMA Dermatol 2022
- [CrossRef] [PubMed] [Google Scholar]
- Hotspot analysis by confocal microscopy can help to differentiate challenging melanocytic skin lesions. PLoS One. 2022;17:e0263819.
- [CrossRef] [PubMed] [Google Scholar]
- Lentigo maligna melanoma mapping using reflectance confocal microscopy correlates with staged excision: A prospective study. J Am Acad Dermatol 2019
- [CrossRef] [Google Scholar]
- In vivo confocal microscopy of basal cell carcinoma: A systematic review of diagnostic accuracy. J Eur Acad Dermatol Venereol. 2015;29:1890-7.
- [CrossRef] [PubMed] [Google Scholar]
- Basal cell carcinoma: Comprehensive clinical and histopathological aspects, novel imaging tools and therapeutic approaches (Review) Exp Ther Med. 2022;23:60.
- [CrossRef] [PubMed] [Google Scholar]
- In vivo confocal laser scanning microscopy imaging of skin inflammation: Clinical applications and research directions. Exp Ther Med. 2019;17:1004-11.
- [CrossRef] [Google Scholar]
- In vivo reflectance confocal microscopy of oral lichen planus. Int J Dermatol. 2019;58:940-5.
- [CrossRef] [PubMed] [Google Scholar]
- Pilot study on reflectance confocal microscopy imaging of lichen planus: A real-time, non-invasive aid for clinical diagnosis. J Eur Acad Dermatol Venereol. 2012;26:1258-65.
- [CrossRef] [PubMed] [Google Scholar]
- The combined role of clinical, reflectance confocal microscopy and dermoscopy applied to chronic discoid cutaneous lupus and subacutus lupus erythematosus: A case series and literature review. Lupus. 2021;30:125-33.
- [CrossRef] [PubMed] [Google Scholar]
- Reflectance confocal microscopy for scarring and non-scarring alopecia real-time assessment. Arch Dermatol Res. 2016;308:309-18.
- [CrossRef] [PubMed] [Google Scholar]
- Reflectance confocal microscopy applied to folliculitis decalvans: Preliminary results of a multicenter study. Skin Appendage Disord. 2020;6:202-6.
- [CrossRef] [PubMed] [Google Scholar]
- Introduction to confocal microscopy. J Invest Dermatol. 2012;132:e3.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical applications of reflectance confocal microscopy in the management of cutaneous tumors. Actas Dermosifiliogr. 2008;99:528-31.
- [CrossRef] [Google Scholar]
- Line scanning, stage scanning confocal microscope (LSSSCM) Biomed Opt Express. 2017;8:3807-15.
- [CrossRef] [PubMed] [Google Scholar]






