Translate this page into:
Demonstration of fungi in superficial mycosis: Skin scraping and potassium hydroxide mount
*Corresponding author: Parvathy Santhosh, Department of Dermatology, Malabar Medical College, Kozhikode, Kerala, India. drparvathysanthosh@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Santhosh P, George M, Nandakumar G. Demonstration of fungi in superficial mycosis: Skin scraping and potassium hydroxide mount. J Skin Sex Transm Dis 2024;6:77-9. doi: 10.25259/JSSTD_31_2022.
Abstract
Skin scraping for demonstration of fungi in superficial mycosis is a commonly performed procedure that aids in the diagnosis of conditions such as dermatophyte infections of the skin and hair, onychomycosis, pityriasis versicolor, and mucocutaneous candidiasis. After taking consent from the patient, specimens are collected onto a black paper and transferred to a glass slide, followed by the addition of potassium hydroxide solution. The slide is examined under the microscope for fungal elements. Careful collection and preparation of specimens are essential for accurate results.
Keywords
Fungal infections
Potassium hydroxide
Dermatophytosis
Pityriasis versicolor
Superficial mycosis
INTRODUCTION
Skin scraping for demonstration of fungi in superficial mycosis is a commonly performed procedure in dermatology departments. It is essential for all dermatology postgraduate trainees to learn the procedure accurately.
INDICATIONS
PROCEDURE
Materials needed
Cotton swab, alcohol for cleaning, blunt scalpel, black paper, paper clip, sticker, glass slide, coverslip, 10% potassium hydroxide (KOH) solution, and Bunsen burner or spirit lamp.
Collection of specimens
The procedure must be explained in detail to the patient and informed written consent must be obtained. Before taking the specimen, the lesions may be cleaned with alcohol or distilled water to remove any topical medicament/powder (that the patient has applied) or any cloth fibers. This would also enable easy sampling of the scales.[1-3]
The scrapings must be collected from the most heavily infected area of the skin or the active edge of the lesion. If the active edge cannot be discerned, scraping from much of the area is sufficient.[1,2]
The skin above the site may be pulled up. The scalpel must be held vertically to the skin and the blunt scalpel edge moved across the skin to obtain scrapings. The scrapings should be collected in a black paper. This enables easy visualization of the skin debris, keeps the specimen dry, and prevents contamination and bacterial overgrowth. The paper must be folded carefully, secured with a paper clip, and labeled appropriately. The details of the patient, exact site of the lesion, and the date and the time of collection of the specimen must be noted. Specimen can also be collected directly on to a glass slide; however, this involves the risk of breakage during transportation. Plastic containers are not suitable as the skin debris adheres to them causing difficulty in removal.[1]
When collected in paper, the specimen must be carefully transferred to a labeled glass slide. One to two drops of KOH solution (10% for skin lesions, 20–40% for thick hyperkeratotic lesions and nails) are added to the collected material. A cover slip is applied and pressed down to obtain a monolayer of cells. However, pressing must be avoided in hair specimens as it can cause distortion and loss of texture. Excess KOH can etch microscopic lenses, hence must be blotted away with small squares of filter paper or tissue paper. For thick, hyperkeratotic lesions, the KOH mounted specimens must be left for 30– 60 minutes for digestion and clearing. In the case of skin and nail specimens, warming gently over a Bunsen burner or a spirit lamp will speed up the process. However, boiling must be avoided, as it may cause artifacts. Nail specimens may take longer to soften and may have to be kept for 12–24 hours. Infected hairs are very delicate and will disintegrate, if heated or left in KOH for too long, which will obscure the arrangement of conidia. If the mount dries, air pockets will form under the coverslip. If that happens, more KOH must be added [Video 1].[1-4]
Video 1:
Video 1:Video demonstrating skin scraping and potassium hydroxide mount.Microscopic examination
The slide is mounted on the light microscope. It is initially viewed under low power objective lens. In this magnification, over-illumination will render the fungal elements invisible. The light should, therefore, be low initially, and the condenser must be kept partially closed. The presence of fungus under low power magnification should be confirmed by examination under a higher power lens (20× or 40×), and here, the illumination can be increased.[1]
Once the procedure is completed, the specimens and materials must be disposed of appropriately, following the guidelines for biomedical waste management [Video 1].[4]
INTERPRETATION OF RESULTS
Dermatophytes
Long, smooth, refractile, branching, and septate hyphal filaments, with or without arthroconidiospores [Figure 1].
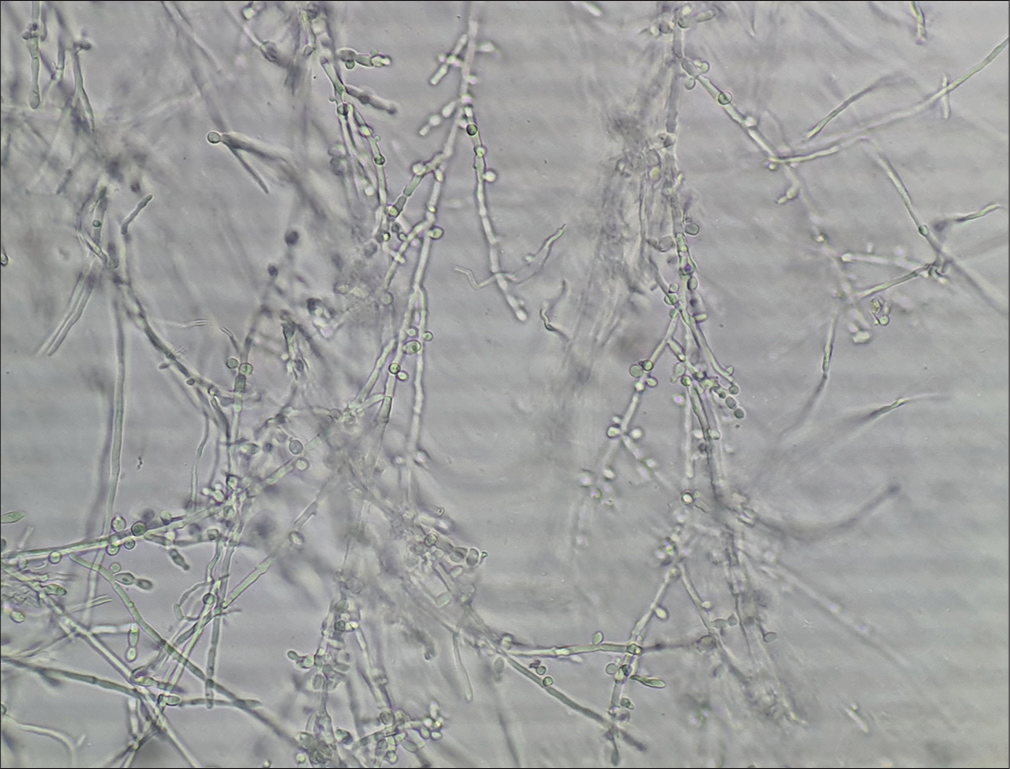
- Refractile, long, smooth, branching, septate hyphal filaments, and arthroconidiospores in dermatophytosis.
Non-dermatophyte molds
Irregular, tortuous, vesiculated, or pigmented hyphal filaments and spores.
Tinea capitis
In ectothrix infections, multiple spores lying in groups outside the hair shaft are observed. Endothrix infections show broken hair shafts invaded by arthrospores, which may show a mosaic pattern or linear longitudinal arrangement of arthrospores inside the shaft, and spores may be seen outside the hair cortex as well.
Candidiasis
Hyaline, oval, budding yeast cells (blastoconidia), and pseudo-hyphal forms are seen.
Pityriasis versicolor
Clusters of thick-walled, spherical yeasts, and short filaments of hyphae with a “banana and grapes” or “spaghetti and meatballs” appearance are seen [Figure 2].[2,3]
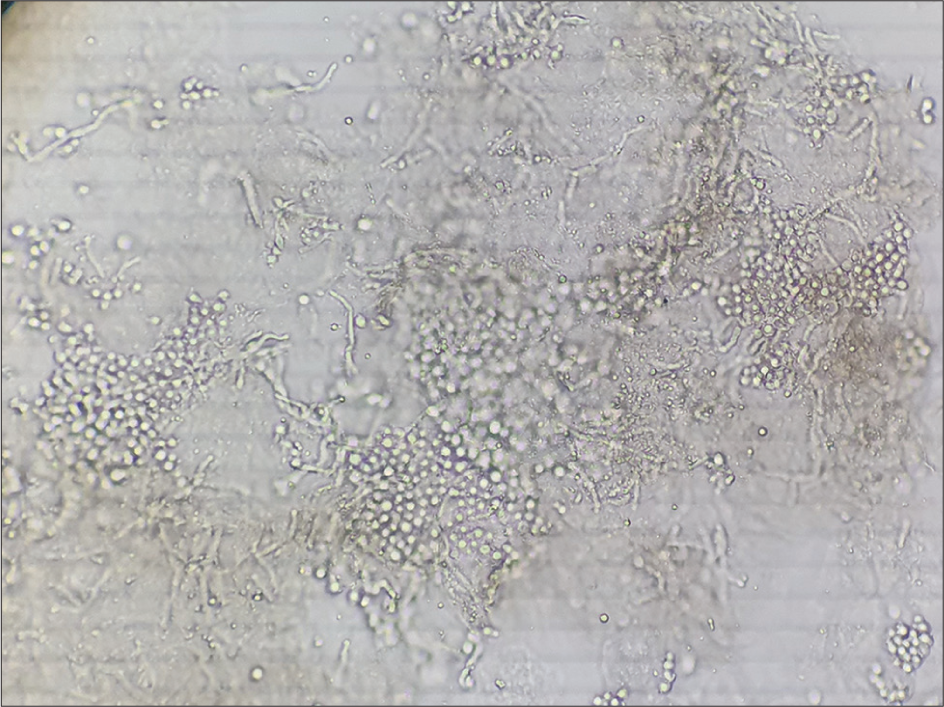
- Clusters of thick-walled, spherical yeasts, and short filaments of hyphae with a “banana and grapes” or “spaghetti and meatballs” appearance in pityriasis versicolor.
CONCLUSION
The successful demonstration of fungus in KOH preparation is chiefly a matter of practice. Careful collection and preparation of specimens are crucial, and a few extra minutes spent to follow the procedure accurately are well worth it. Artifacts can confuse the beginner – such as air bubbles, mosaic fungus (cholesterol-forming polygonal deposits around cells), fibers, and crystals. However, ardent practice renders it progressively easier to find and distinguish the various fungi.
Acknowledgment
The authors express sincere gratitude to Dr. V. Mohamed Althaf, Junior Resident, Department of Dermatology, Government Medical College, Kozhikode for the images of the potassium hydroxide mount. Background music in the video is under a creative commons license (Anomalous Hedges by The Mini Vandals, https://www.youtube.com/watch?v=5DXfB_Qtqoo).
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
Dr. Parvathy Santhosh and Dr. Mamatha George are on the editorial board of the Journal.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Video available online at:
Financial support and sponsorship
Nil.
References
- Fungal infections In: Burns T, Breathnach S, Cox N, Griffiths C, eds. Rook's Textbook of Dermatology (9th ed). Hoboken, NJ: Wiley-Blackwell; 2016. p. :32.1-96.
- [Google Scholar]
- Skin scraping and a potassium hydroxide mount. Indian J Dermatol Venereol Leprol. 2006;72:238-41.
- [CrossRef] [PubMed] [Google Scholar]
- Mount the menace! Potassium hydroxide in superficial fungal infections. Indian J Paediatr Dermatol. 2020;21:343-6.
- [CrossRef] [Google Scholar]
- Biomedical waste management in India: Critical appraisal. J Lab Physicians. 2018;10:6-14.
- [CrossRef] [PubMed] [Google Scholar]






