Translate this page into:
Diffuse facial melanosis – An overview of etiology and dermoscopic findings
-
Received: ,
Accepted: ,
How to cite this article: Dharman BK, Sridhar S. Diffuse facial melanosis – An overview of etiology and dermoscopic findings. J Skin Sex Transm Dis 2020;2(2):86-93.
Abstract
Facial pigmentary disorder is a common condition in dark-skinned individual which causes significant psychological morbidity to the patients. Some of the well-defined causes of facial melanoses include melasma, Riehl’s melanosis, lichen planus pigmentosus, erythema dyschromicum perstans, and poikiloderma of Civatte. However, most of these conditions share many clinical and histopathological features in common. Sunlight exposure is one of the most common etiological factors, but application of irritant and photochemical substances also plays a role. Treatment of facial pigmentation is difficult and involves mainly the usage of various skin lightening agents acting at different levels of melanogenesis such as hydroquinone, chemical peels, and lasers. There is no universally effective specific therapy – existing agents have varying degrees of efficacy and relapses are frequent. Dermoscopy has been used mostly in the evaluation of pigmented lesions which have shown superiority over clinical examination and is of immense help to the clinicians for the appreciation of subtle features which are invisible to the naked eye.
Keywords
Melasma
Erythema dyschromicum perstans
Facial melanoses
Hydroquinone
Lichen planus pigmentosus
INTRODUCTION
Hypermelanosis involving predominantly the face and neck is relatively common and often presents a complex diagnostic problem. Genetic and racial factors are important, the increased pigmentation occurring more frequently in those with dark skins, especially those of Middle Eastern or Asian descent.[1,2]
Endocrine factors play a major role in melasma and are implicated to some degree in other melanoses.[3] External agents (light and photodynamic chemicals) are essential factors in the occupational melanoses but are also implicated in photocontact dermatitis (Riehl melanosis), and erythromelanosis and poikiloderma of Civatte[4] dermoscopy in daily clinical practice help the clinicians to observe the subtle features and to make a precise diagnosis.[5,6]
Based on the location of melanin as identified by color of lesion, accentuation of color under Wood’s light and histopathology, though the correlation between Wood’s lamp findings and histopathology is less than satisfactory, three types of hypermelanosis are identified: [1]
Brown hypermelanosis: Wherein excess melanin is in basal and suprabasal (rarely throughout epidermis including the horny) layers and the pigmentation is accentuated under Wood’s lamp
The increased epidermal melanin can be a result of:
Melanotic hypermelanosis: Due to increased melanin production by normal number of melanocytes
Melanocytic hypermelanosis: Due to the increased number of melanocytes.
Blue hypermelanosis (ceruloderma): Wherein excess melanin is in dermis and the pigmentation is not accentuated under Wood’s lamp
Ceruloderma is due to:
Increased transfer of melanin from epidermis to dermis (pigmentary incontinence): Melanin granules accumulate within melanophages or may be free in the extracellular matrix of the dermis
Production of melanin: By ectopic dermal melanocytes
Binding of melanin: To exogenous pigments deposited in the dermis.
Mixed hypermelanosis: Due to increased epidermal and dermal melanin.[1]
MELASMA
Melasma is a common acquired disorder characterized by symmetric, hyperpigmented patches with an irregular outline, occurring most commonly on the face. Exacerbating factors include sun exposure, pregnancy, thyroid disorders, and drugs such as phenytoin and oral contraceptive pills.[7]
Light to dark brown or brown-gray patches with irregular borders appears primarily on the face. The areas of hypermelanosis are distributed symmetrically in three classic patterns: (1) Centrofacial (most common), involving the forehead, cheeks, nose, upper lip (sparing the philtrum and nasolabial folds), and chin; (2) malar, affecting the cheeks and nose; and (3) mandibular, along the jawline.
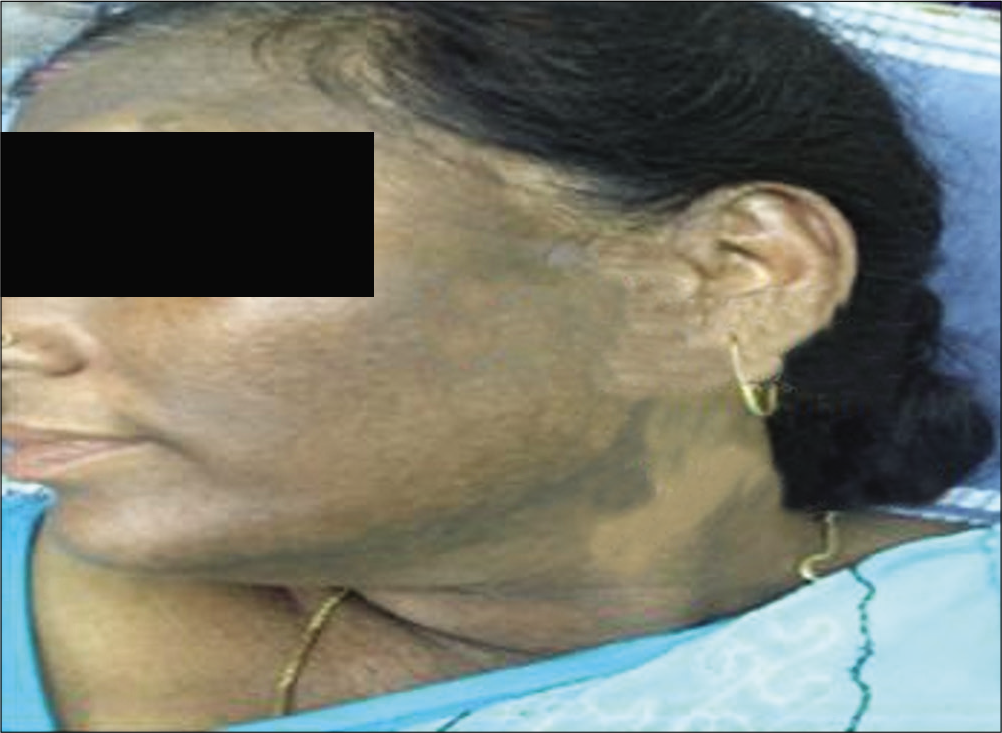
Melasma has classically been subdivided into four types based on the primary location of the pigment: Epidermal [Figure 1], dermal [Figure 2], mixed, or indeterminate (e.g., in patients with very dark skin pigmentation). In theory, lesions with increased epidermal melanin are accentuated and those with increased dermal melanin become less obvious (i.e., blend with uninvolved skin) with Wood’s lamp examination.[7]
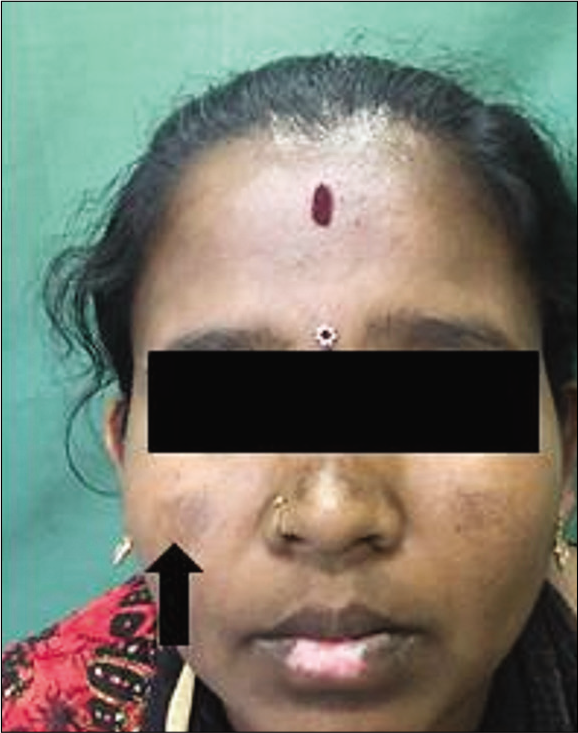
- Epidermal melasma.

- Dermal melasma.
Sun protection is central to management. Epidermal pigmentation is known to be more responsive to topical treatment than dermal pigmentation. Hypopigmenting agents such as hydroquinone (HQ), tretinoin, azelaic acid, rucinol, and kojic acid are helpful when used for prolonged periods.[8] The so-called Kligman formula is a popular combination of HQ, tretinoin, and a mild topical corticoid.[9] Chemical peels and laser therapy may be helpful in the treatment of melasma but can also result in further unwanted hyperpigmentation. Sometimes, melasma slowly disappears after discontinuation of the hormonal stimulus and/or careful sun avoidance.
RIEHL’S MELANOSIS/PIGMENTED COSMETIC DERMATITIS
Riehls melanosis is attributable to phototoxic reaction develops after skin contact with photoactive agents. Tar derivatives, cosmetics, and fragrances[10] are suspected to be the cause and it is found to be more common in middle-aged women.
Brownish-gray pigmentation develops quite rapidly over the greater part of the face but is more intense on the forehead and temples. Smaller pigmented macules, often perifollicular, lie beyond the indefinite margin. The pigmentation may extend to the chest, neck, and scalp, and occasionally involves the hands and forearms. Horny plugs fill the follicles and there may be some scaling.[6] Causative contact identified and avoided.[11]
POIKILODERMA OF CIVATTE/ ERYTHROMELANOSIS INTERFOLLICULARIS COLLI
Poikiloderma of Civatte presents as mottled pigmentation (atrophy, telangiectasia, hyper and hypo-pigmentation) which typically appears on the sides of the face and neck and on the upper anterior chest after years of repeated ultraviolet exposure.
Exposure to light and photodynamic substances in cosmetics is influencing factors.[12] It is more common in middle-aged women. Poikilodermatous changes develop symmetrically on the sides of the face, neck, and upper aspect of the chest with hyperpigmentation, telangiectasia, and dermal atrophy.
The submandibular and submental areas are spared, thus implicating sunlight in the pathogenesis of this condition.[6] Patients should be advised to use high sun protection factor sunscreens and to avoid undue sun exposure. Laser therapy with tunable dye laser may be effective, but care is needed as it may also cause scarring and may even worsen the appearance.[13]
ERYTHROMELANOSIS FOLLICULARIS OF THE FACE AND NECK
Erythromelanosis follicularis of the face and neck presents with a reddish-brown discoloration affecting the preauricular and maxillary regions, in some cases spreading to the temples and lateral sides of the neck and trunk, with symmetrical distribution and sharp demarcation from normal skin.[6]
It primarily affects young or middle-aged males[14,15] and sometimes in adult females.[16] It is characterized by reddish-brown pigmentation, telangiectasia studded with pale follicular papules. Alopecia characteristically affecting fine vellus hair and sparing the terminal hair is seen. The pigmentation spreads gradually and may persist for a long time. Not influenced by treatment.[11]
ERYTHEMA DYSCHROMICUM PERSTANS (EDP)/ASHY DERMATOSIS OF RAMIREZ, ERYTHEMA CHRONICUM FIGURATUM MELANODERMICUM
The etiology of EDP is unknown, but anecdotal reports have incriminated exposure to ammonium nitrite, radiographic contrast media and chlorothalonil, intestinal whipworm infestation, cobalt allergy, and HIV infection. In Mexican Mestizo patients, HLA-DR4 is associated with a genetic susceptibility to develop EDP. The relation of EDP to lichen planus (LP) is uncertain, both have several clinical, histological, and immunohistochemical similarities and often coexist making some authors consider EDP a variant of LP.
EDP presents as numerous asymptomatic, gradually enlarging and coalescing, persistent, and macules of variable sizes [Figure 3]. Initially having an erythematous hue and an elevated dusky border (not always noted), lesions eventually become pigmented. Initially localized, lesions eventually cover extensive areas of face, trunk, and limbs.[1]

- Erythema dyschromicum perstans.
No specifically established treatment is present. Various therapies in the form of sun protection, chemical peels, corticosteroids, antibiotics, vitamins, griseofulvin, isoniazid, and chloroquine have been tried without much benefit.[11]
LP PIGMENTOSUS (LPP)
Although the exact etiology of LPP is not known, cosmetics, including fragrances, hair dyes, and mustard oil, have been incriminated. LPP is characterized by generally asymptomatic (sometimes itchy) and diffuse (less frequently reticular, blotchy, or perifollicular) hyperpigmented dark-brown to slate gray to black macules present mostly overexposed areas [Figure 4] and flexures. The lesions lack the erythematous border of EDP. The clinical association of this entity with lesions of classical LP in about a third of patients and demonstration of colloid bodies on histopathology prompted Bhutani et al.,[17] to consider LPP a macular variant of LP and very similar to EDP. Although the mucous membranes are characteristically spared, some patients may have LP-like lesions.[1]
- Lichen planus pigmentosus.
PERIORBITAL MELANOSIS (POM)
Factors incriminated in etiology of POM include dermal melanin deposition, post-inflammatory hyperpigmentation (PIH) (atopic or contact allergic dermatitis), shadowing from lax skin, and infraorbital swelling have been incriminated. POM is characterized by variegated brown to almost black discoloration around the eyes. Familial periorbital hyperpigmentation is determined by an autosomal dominant gene[18] and in one study, POM was found to be an extension of pigmentary demarcation lines over the face.[19]
EXOGENOUS OCHRONOSIS (EO)
EO, a rare complication of HQ, develops after prolonged use of high concentrations in dark-skinned patients and rapidly after use of even 2% in white-skinned. It presents with diffuse pigmentation, which under high magnification especially when using polarized light, is characterized by tiny, <1-mm sooty blue macules in a reticulate pattern. Lesions are present in the HQ-treated photo-exposed areas, namely, cheeks, forehead and temporal and periorbital skin with less frequent involvement of nasal, peribuccal, and chin areas [Figure 5]. Biopsy shows banana-shaped yellow-brown granules in and around collagen bundles along with giant cells and melanophage rich granulomas. Improvement occurs only very slowly (if at all) on withdrawal of HQ.[1]

- Exogenous ochronosis.
ADDISONIAN PIGMENTATION
It is diffuse hyperpigmentation more prominent in the sun-exposed areas, flexures (e.g., axillae and popliteal fossae), palmar and plantar creases, and areas subject to friction. Increased genital pigmentation is also common. Increased pigmentation of scars also seen.[11] Oral pigmentation involving gums and mucosa is a prominent feature.[20]
NEVUS OF OTA (NOO)
NOO or oculodermal melanocytosis more common in Japanese in women with onset either in the perinatal period (50%) or around puberty (30%).[21]
PATHOGENESIS
NOO represents aborted embryonic migration of melanocytes from neural crest to epidermis. Late pubertal onset is explained by pigmentation of the amelanotic nevoid cells present at birth by adolescent spurt of sex hormones.
CLINICAL FEATURES
NOO is characterized by speckled or mottled coalescing blue-gray pigmentation of the area supplied by ophthalmic and maxillary divisions of trigeminal nerve [Figure 6]. It is usually unilateral (90%). In addition to skin, pigmentation of NOO may involve oral mucosa and the eye (conjunctiva, sclera, retrobulbar fat, cornea, and retina) in which two levels of pigmentation – brown of conjunctiva and blue of sclera (often not overlapping) are clearly discernible. Based on the extent, NOO is classified into:

- Nevus of Ota.
Type I (mild):
IA: Mild orbital type: On upper and lower eyelids, periocular, and temple region
IB: Mild zygomatic type: On the infrapalpebral fold, nasolabial fold, and zygomatic region
IC: Mild forehead type: On forehead only
ID: On ala nasi only.
Type II (moderate): On upper and lower eyelids, periocular, zygomatic, cheek, and temple regions.
Type III (severe): On scalp, forehead, eyebrow, and nose.
Type IV (bilateral type): Bilateral.
VARIANTS
Hori nevus,[22] or acquired bilateral nevus of Ota-like macules (ABNOM) is probably a distinct variant seen in Koreans and Japanese. Unlike NOO, it has a late onset in adulthood, spares the mucosae, and is clinically characterized by bilaterally symmetrical speckled or confluent brownish-blue or slate gray pigmentation over the malar regions, temples, root of the nose, alae nasi, the eyelids, and forehead. In cases of ABNOM confined only to the malar area or forehead, diagnosis is difficult because it mimics the centrofacial type of melasma. Without careful examination of alae nasi, forehead, and eyelids, brown ABNOM looks just like melasma, though histologically, fusiform-elongated dendritic melanocytes and melanophages are scattered among collagen bundles. The differentiation is essential because both are treated differently – melasma with topical agents while ABNOM with lasers.
COURSE
Although NOO persists, it is only rarely complicated by melanoma and is anecdotally associated with ipsilateral glaucoma.
ACANTHOSIS NIGRICANS
Acanthosis nigricans is characterized by hyperpigmented, velvety plaques of body folds may also involve the face as well. Symptomatic treatments include topical retinoids and keratolytics.[1] It is usually associated with weight gain but other underlying conditions leading to insulin resistance should be ruled out. Predominantly lateral aspect of the face, forehead, and periorbital areas is involved in facial acanthosis nigricans.[23]
DRUG-INDUCED PIGMENTATION
Skin pigmentation may be induced by a wide variety of drugs. Several mechanisms are involved in drug-induced changes of pigmentation of the skin. These include increased melanin synthesis, increased lipofuscin synthesis, deposition of drug related material, and PIH.[24] Amiodarone can produce blue-gray pigmentation in sun-exposed areas due to accumulation of a lipid-like substance in macrophages. Some of these patients display photosensitivity. Hydroxychloroquine may give rise to a yellow-brown to bluish-gray pigmentation on the face, neck, lower extremities, and forearms after several years of intake, due to deposition of a drug melanin complex in the dermis. Chlorpromazine and related phenothiazines can produce a bluish-gray pigmentation, especially in sun-exposed areas, and pigmentation of the conjunctivae. Minocycline can induce skin hyperpigmentation, as well as pigmentation of the nails, sclerae, oral mucosa, thyroid, bones, and teeth.[8]
PIH
PIH is a common condition caused by numerous preceding cutaneous insults such as drug and phototoxic reactions, infections, physical injury or trauma, allergic reactions, and inflammatory diseases.
Clinically, PIH consists of a macular hyperpigmentation at the site of inflammation. It is far more common and persistent in darker skin types (Fitzpatrick Types III–VI) and can be characterized by epidermal as well as dermal melanotic hypermelanosis. A Wood’s lamp examination can determine depth of the hyperpigmentation. Management of PIH remains difficult, although epidermal PIH often shows slow spontaneous fading.[8]
PERIBUCCAL PIGMENTATION OF BROCQ
This occurs predominantly in middle-aged women.[25] A photodynamic substance in cosmetics is probably responsible. Diffuse brownish-red pigmentation develops symmetrically around mouth but spares a narrow perioral ring. It may extend up the center of the face to the forehead, angles of jaw, and temples. Erythema may fluctuate, but pigmentation is persistent. Similar PIH is seen in patients with perioral dermatitis and may be the result of topical steroid therapy.[26]
DERMOSCOPY
Dermatoscopy is an in vivo noninvasive technique used to examine pigmented and amelanotic skin lesions. The technique is performed using a handheld self-illuminating device called dermatoscope that visualizes features present under the skin surface that is not normally visible to unaided eye. The images from the dermatoscope can be digitally photographed or recorded for future reference. Nonpolarized dermatoscopy requires contact with the skin surface and interface fluid between glass and skin surface.[27] Polarized light penetrates deeper than the nonpolarized light and does not require contact fluids. Skin surface microscopy was first performed in the early 20th century by Johann Saphier (1920) using a binocular microscope having an inbuilt light source. Leon Goldman (1951), also known as father of dermatoscopy, used the technique for the evaluation of pigmented lesions. The essential components of a dermatoscope include as follows:
Illumination system
Achromatic lens
Contact plate
Power supply.
NONPOLARIZED VERSUS POLARIZED DERMOSCOPY
Nonpolarized dermatoscopy requires contact with the skin surface and interface fluid, whereas polarized dermatoscopy neither requires contact nor fluid. Nonpolarized dermatoscopy helps in better visualization of superficial structures such as comedo-like openings, milia-like cyst, crypts, fissures, and scales; deeper structures such as white, shiny streaks, vessels, and pigment network are more conspicuous with polarized dermatoscopy.[28] Hence, nonpolarized and polarized dermatoscopy techniques are complementary to each other, with structures more apparent with one mode not being clearly visible in the other mode. These structures blink when toggled between both these modes in hybrid dermatoscopes possessing both these modes.[29]
MELASMA
A pseudoreticular pigment network with concave borders (jelly sign), diffuse light-to-dark brown background with sparing of the periappendageal region (follicular and sweat gland openings), brown granules, and globules, including arcuate and annular structures is seen on dermoscopy [Table 1].[30] Epidermal melasma demonstrates blotchy brownish reticular pattern showing multiple granules and globules of dark brown color superimposed on reticular pattern. Dermal melasma shows grayish-brown or grayish-black pigmented specks or arcuate, star shaped, honeycomb, and annular structures mainly in perifollicular location sparing follicles. Mixed melasma shows diffuse reticular pigmentation of dark brown or blackish pigmentation of various size. Granules and globules of dark brown color also seen in perifollicular area sparing follicles [Figure 7].[31]
| Dermatoscopic features | Melasma | lichen planus pigmentosus | Riehl’s melanosis | Exogenous ochronosis | Ashy dermatosis |
|---|---|---|---|---|---|
| Background | Light brown-to-dark brown pigmentation | Blue-gray pigmentation | Brown-to-gray pigmentation | Dark hyperpigmentation | Dark brown pigmentation |
| Reticular network | Brown to black | Nonuniform accentuation | Pseudonetwork | Dark brown to black | Uniform accentuation |
| Pigment deposition–gray-to-brown dots and globules | Dark brown-colored globules/dots/blotches additionally seen | Slate gray dots with regular distribution of pigment seen | Uneven with clustering of pigment dots | Blue-gray dots and globules with a caviar-like appearance | Bluish-gray pigment evenly spaced |
| Pattern | The pigmentation pattern becomes pseudoreticular with diffuse dark brown-to-grayish pigmentation with sparing of follicular openings | Hem-like pattern in a typical case, especially in nonfacial lesions | None | Obliteration of follicular opening. Elongated and curvilinear worm-like structures conjoined together in a reticulate pattern of ochronosis | Rounded, linear, granular, discrete bluish-gray pigment is seen and at places tending to form curvilinear pattern |

- Dark brown background with brown dots and globules in pseudoreticular pattern showing perifollicular sparing in melasma (Derma India, polarized mode, ×10) – picture courtesy Dr. Bindu V, Additional Professor, Govt. Medical College, Kozhikode.
LPP
Dermoscopic examination of LPP shows the absence of Wickham striae and vascular features [Table 1]. It is characterized by the presence of pigment pattern such as slate gray-to-blue dots and globules which are not uniformly accentuated. Deposition of pigment around acrosyringium is accentuated. Perifollicular pigment deposition noted [Figure 8]. “Hem-like” pigment pattern has also been described and background color on dermoscopy is brown.[31-33]

- Diffusely distributed brown dots and globules showing perifollicular accentuation in some areas in lichen planus pigmentosus (DermLite DL4n, polarized mode, ×10) – picture courtesy Dr. Puravoor Jayasree, Consultant Dermatologist, Medical Trust Hospital, Cochin.
RIEHL’ S MELANOSIS
Pigmented contact dermatitis on dermoscopy shows uneven distribution of brown-to-gray-colored dots and globules with pseudo network of reticular network and erythematous background [Table 1].[30]
EO
Dermoscopy reveals blue-gray dots and globules with a caviar-like appearance with dark brown reticular network and background of dark hyperpigmentation with obliteration of follicular opening. Elongated and curvilinear worm-like structures conjoined together in a reticulate pattern of ochronosis.[34] Multiple thick globular structures over melasma patches seen with multiple short and thin arciform structures. Confetti-like depigmentation seen as white dots [Figure 9] and telangiectasias seen as red dots [Table 1].[30]

- Brown amorphous areas, confetti-like depigmentation seen as white dots and obliteration of follicular openings in exogenous ochronosis (DermLite 4, polarized mode, ×10).
ASHY DERMATOSIS
Uniform reticular pattern is accentuated. Reticular pattern is complete at places, whereas at other places they disintegrate into more discrete, speckled, granular, linear, angulated bluish-gray deposits. Pigment surrounding acrosyringeal openings is blunted. Rounded, linear, granular, discrete bluish-gray pigment is seen which is evenly spaced and at places tending to form curvilinear pattern. Pigment clusters are limited by skin surface markings and do not cross them [Table 1].[32]
PERIORBITAL HYPERMELANOSIS
The different patterns of pigmentation noted are blotches, exaggerated pigment network, coarse speckled, fine speckled, and globules. Pattern of vasculature included telangiectases and superficial dilated vessels. Patterns of skin changes included atrophy and exaggerated skin markings.[35]
CONCLUSION
Diffuse facial pigmentation is a serious concern especially in skin of color. There are no specific guidelines for the management of facial melanoses. The diagnosis and management is challenging because of their heterogenous nature. Picking up the definite clinical clues and alert history taking will help in arriving a diagnosis. Dermatoscope is a tool of immense help to a dermatologist for a non invasive and in vivo diagnosis of facial pigmentary conditions.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Facial melanoses: Indian perspective. Indian J Dermatol Venereol Leprol. 2011;77:552-63.
- [CrossRef] [PubMed] [Google Scholar]
- Topical treatment of melasma. Indian J Dermatol. 2009;54:303-9.
- [CrossRef] [PubMed] [Google Scholar]
- Hyperpigmentation and melasma. J Cosmet Dermatol. 2007;6:195-202.
- [CrossRef] [PubMed] [Google Scholar]
- Acquired pigmentary disorders In: Griffiths CE, Barker J, Blelker T, Chalmers R, Creamer D, eds. Rook's Textbook of Dermatology (9th ed). West Sussex: John Wiley and Sons Ltd; 2016. p. :88.10-25.
- [Google Scholar]
- Dermoscopy of pigmented skin lesions-a valuable tool for early diagnosis of melanoma. Lancet Oncol. 2001;2:443-9.
- [CrossRef] [Google Scholar]
- Principles and technique of dermoscopy and videodermoscopy. In: Khopkar US, ed. Dermoscopy and Trichoscopy in Diseases of the Brown Skin-atlas and Short Text (1st ed). New Delhi: Jaypee Publications; 2012. p. :1.
- [CrossRef] [Google Scholar]
- Disorders of hyperpigmentation In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology (4th ed). Amsterdam: Elsevier; 2018. p. :1119.
- [Google Scholar]
- Hypomelanoses and hypermelanoses In: Katz SI, Gilchrest BA, Paller A, Leffel DJ, Wolff K, Goldsmith LA, eds. Fitzpatrick's Dermatology in General Medicine (8th ed). New York: McGraw-Hill Professional; 2012. p. :819-24.
- [Google Scholar]
- A new formula for depigmenting human skin. Arch Dermatol. 1975;111:40-8.
- [CrossRef] [Google Scholar]
- Riehl's melanosis: Pigmented contact dermatitis caused by fragrances. J Am Acad Dermatol. 1989;21:1057-60.
- [CrossRef] [Google Scholar]
- Disorders of hyperpigmentation In: Sacchidanand S, Oberai C, Inamadar AC, eds. IADVL Textbook of Dermatology (4th ed). Mumbai: Bhalani Publishing House; 2015. p. :1327-57.
- [Google Scholar]
- Management of facial hyperpigmentation. Am J Clin Dermatol. 2000;1:261-8.
- [CrossRef] [PubMed] [Google Scholar]
- Flashlamp-pumped pulsed dye laser therapy for poikiloderma of civatte. J Dermatol Surg Oncol. 1990;16:12-6.
- [CrossRef] [PubMed] [Google Scholar]
- Erythromelanosis follicularis faciei et colli. A case report. J Am Acad Dermatol. 1981;5:533-4.
- [CrossRef] [Google Scholar]
- Erythromelanosis follicularis faciei et colli. Case reports. Br J Dermatol. 1980;102:323-5.
- [CrossRef] [PubMed] [Google Scholar]
- Erythromelanosis follicularis faciei et colli in women. J Am Acad Dermatol. 1995;32:863-6.
- [CrossRef] [Google Scholar]
- Periorbital hyperpigmentation. An overlooked genetic disorder of pigmentation. Arch Dermatol. 1969;100:169-74.
- [CrossRef] [PubMed] [Google Scholar]
- Periorbital melanosis is an extension of pigmentary demarcation line-F on face. Indian J Dermatol Venereol Leprol. 2007;73:323-5.
- [CrossRef] [PubMed] [Google Scholar]
- Disorders of pigmentation: An expanding area for research. Sri Lanka J Dermatol. 2002;6:1-5.
- [Google Scholar]
- Acquired, bilateral naevus of Ota-like macules. J Am Acad Dermatol. 1984;10:961-4.
- [CrossRef] [Google Scholar]
- Drug-induced skin pigmentation. Epidemiology, diagnosis and treatment. Am J Clin Dermatol. 2001;2:253-62.
- [CrossRef] [PubMed] [Google Scholar]
- Report on a case of erythrose peribuccale de Brocq. Australas J Dermatol. 1954;2:513.
- [CrossRef] [PubMed] [Google Scholar]
- Abnormal facial pigmentation associated with prolonged use of topical corticosteroids. Scott Med J. 1975;20:277.
- [Google Scholar]
- Dermatoscopy: Physics and principles. Indian J Dermatopathol Diagn Dermatol. 2017;4:27-30.
- [CrossRef] [Google Scholar]
- Differences between polarized light dermoscopy and immersion contact dermoscopy for the evaluation of skin lesions. Arch Dermatol. 2007;143:329-38.
- [CrossRef] [PubMed] [Google Scholar]
- Melasma. In: Khopkar US, ed. Dermoscopy and Trichoscopy in Diseases of the Brown Skin-atlas and Short Text (1st ed). New Delhi: Jaypee Publications; 2012. p. :54-7.
- [CrossRef] [Google Scholar]
- Lichen planus pigmentosus vs ashy dermatosis-through a dermoscope In: Khopkar US, ed. Dermoscopy and Trichoscopy in Diseases of the Brown Skin-atlas and Short Text (1st ed). New Delhi: Jaypee Publications; 2012. p. :138-51.
- [Google Scholar]
- Importance of dermatoscopy in diagnosing exogenous ochronosis. In: Khopkar US, ed. Dermoscopy and Trichoscopy in Diseases of the Brown Skin-atlas and Short Text (1st ed). New Delhi: Jaypee Publications; 2012. p. :66-9.
- [CrossRef] [Google Scholar]
- Clinical and dermoscopic evaluation of periorbital hyperpigmentation. Indian J Dermatopathol Diagn Dermatol. 2018;5:42-7.
- [CrossRef] [Google Scholar]






