Translate this page into:
Epidermal growth factor receptor inhibitor Geftinib-induced erosive pustular dermatosis of the scalp – A report with a brief review of the literature
*Corresponding author: Pradeep S. Nair, Department of Dermatology and Venereology, Government Medical College, Thiruvananthapuram, Kerala, India. dvmchtvm@yahoo.co.in
-
Received: ,
Accepted: ,
How to cite this article: Francis A, Nair PS, Bindu RS. Epidermal growth factor receptor inhibitor Geftinib-induced erosive pustular dermatosis of the scalp – A report with a brief review of the literature. J Skin Sex Transm Dis 2024:6:37-40. doi: 10.25259/JSSTD_41_2023.
Abstract
Gefitinib is an epidermal growth factor receptor inhibitor used in the treatment of non-small cell carcinoma of the lungs, adenocarcinoma of the lungs, and solid malignancies of the head and neck region. A 65-year-old female patient with non-small cell carcinoma lung with bone metastasis was started on gefitinib monotherapy after which the patient noticed papulo-pustular lesions on the scalp, later leading to loss of hair. Dermatological examination revealed erythema, pustules with crusting, along with matting of the hair on the frontoparietal area with some areas of hair loss. Trichoscopic examination showed the absence of follicular openings indicating scarring alopecia. Skin biopsy showed perivascular mixed infiltrate of lymphocytes and neutrophils and absent hair follicles, consistent with gefitinib-induced erosive pustular dermatosis of the scalp, a rare entity.
Keywords
Gefitinib
Erosive pustular dermatosis
Scarring alopecia
INTRODUCTION
Gefitinib is an epidermal growth factor receptor (EGFR) inhibitor used in the treatment of non-small cell carcinoma of the lungs, adenocarcinoma of the lungs, and solid malignancies of the head and neck region.[1] EGFRs are not only found on cancer cells but are also expressed on the skin, sweat glands, and follicular epithelium. They are also involved in cell differentiation, proliferation, migration, angiogenesis, and apoptosis. Therefore, their blockage can cause disorders of skin and hair leading to a myriad of cutaneous adverse reactions such as acneiform lesions, pruritus, xeroderma, and dermatophytosis and paronychia.[2] Gefitinib therapy can rarely lead to pustular erosions on the scalp resulting in scarring or non-scarring alopecia. Generally, these reactions due to gefitinib are not serious enough to stop therapy. We are reporting a case of gefitinib-induced erosive pustular dermatosis (EPD) of the scalp leading to scarring alopecia.
CASE REPORT
A 65-year-old female patient with one-year history of non-small cell carcinoma of the lung with bone metastasis was started on gefitinib monotherapy at a dose of 250 mg once daily. Four months after therapy, the patient noticed papulo-pustular lesions on the scalp, which first started on the frontal region and then spread further, later leading to loss of hair. The lesions were associated with slight tenderness. There was no history of diabetes. There was no history of trauma, procedures, or radiotherapy in the affected area. Dermatological examination revealed erythema, pustules with crusting, along with matting of the hair on the frontoparietal area with some areas of hair loss [Figure 1]. There was no evidence of pediculosis or seborrhea. Trichoscopic examination showed milky red areas, white structureless areas, follicular crusting, keratotic plugging, and the absence of follicular openings indicating scarring alopecia [Figure 2]. Systemic examination was unremarkable.
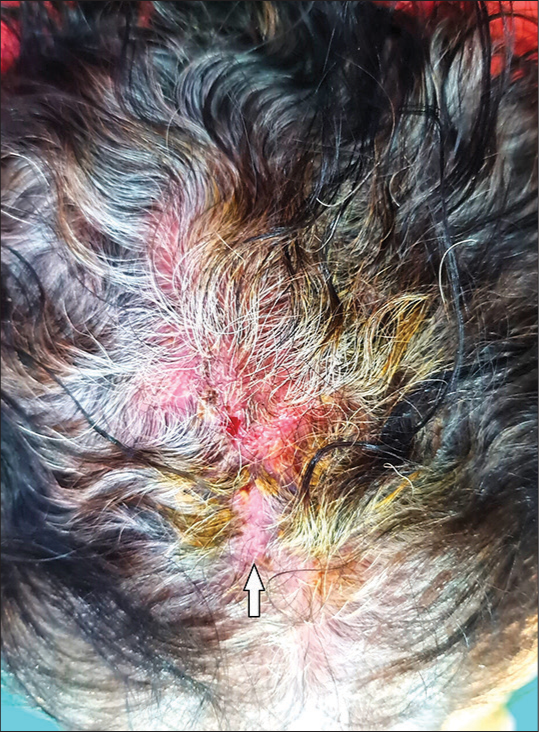
- Erythema, erosions, pustules, and crusting with an area of hair loss (arrow) on the frontoparietal region of the scalp.
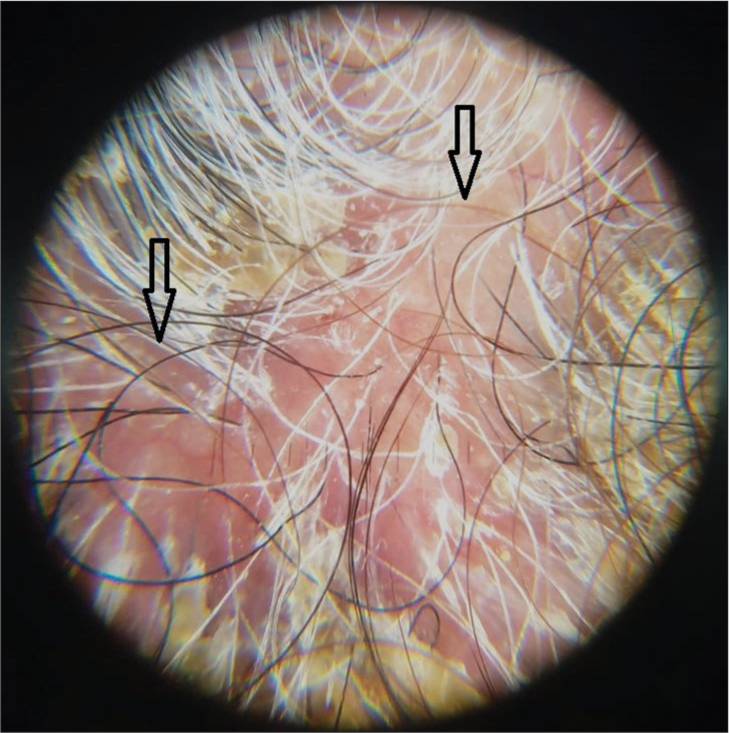
- Trichoscopy showing milky red areas with loss of hair follicles (arrow), Dermalite 10, non polarised ×10.
Hemogram and sugar levels were normal. Viral markers for human immunodeficiency virus, hepatitis B and C were negative. Bacterial culture from the lesions yielded moderate growth of Staphylococcus aureus sensitive to cloxacillin. Histopathological examination of a punch biopsy taken from the parietal scalp lesion showed epidermis with mild hyperkeratosis, irregular acanthosis, and ulceration in one region with the absence of hair follicles in the dermis [Figure 3]. The superficial and mid dermis showed a moderate mixed inflammatory infiltrate composed of lymphocytes, plasma cells, and neutrophils with no hair follicles in the dermis in spite of the biopsy being taken from the scalp [Figure 4]. The patient was treated with cloxacillin and mupirocin due to the culture of S. Aureus, followed by clobetasol lotion. The crusting reduced, but new pustules and papules which were sterile on culture, continued to develop. In view of the progression of the disease, the patient was advised to discontinue gefitinib. After discontinuing gefitinib therapy, the pustular lesions disappeared within a period of 2 weeks, but there was no regrowth of hair in the area. After 2 months of follow-up, the patient had no new lesions. The patient was not on any alternate therapy and was being reevaluated for further therapy.
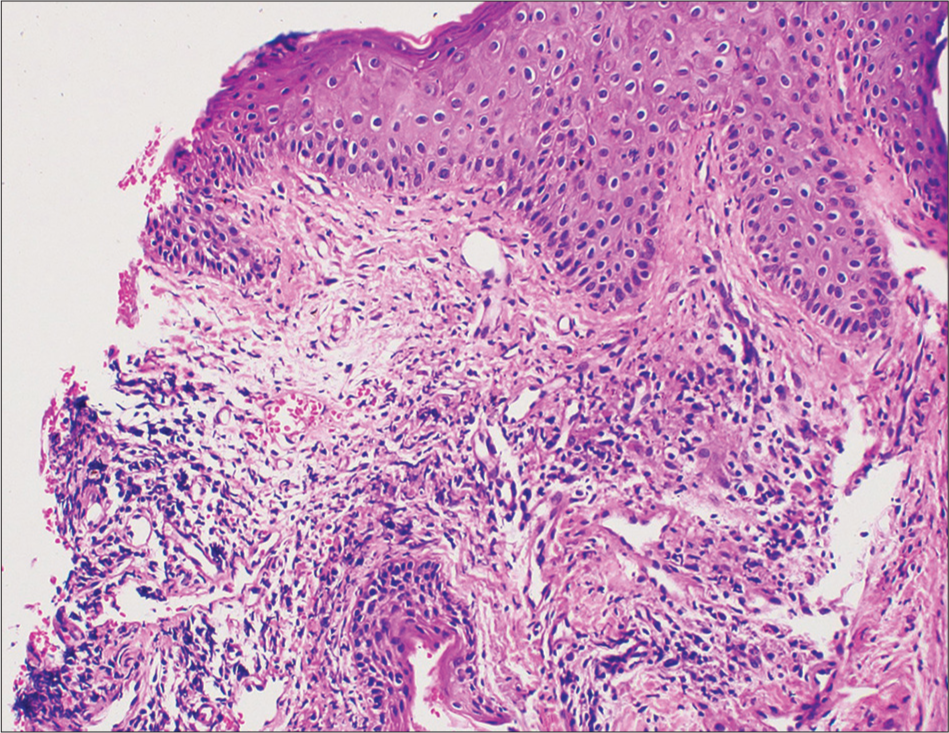
- Skin biopsy showing epidermis with mild hyperkeratosis, irregular acanthosis, and ulceration with no hair follicles in the dermis, (H&E ×200).
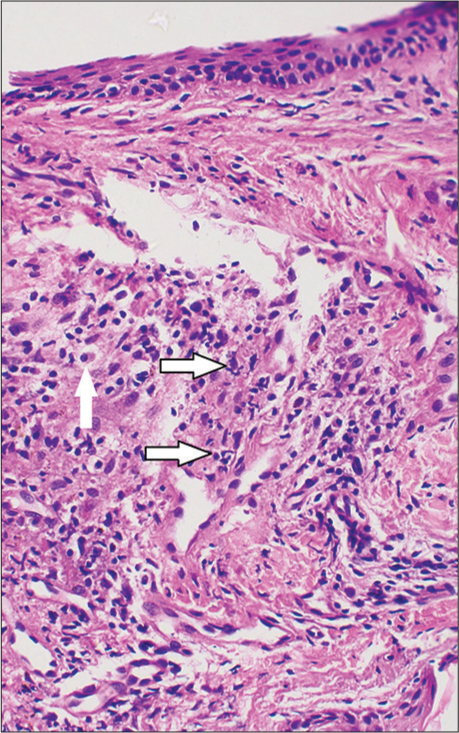
- Skin biopsy showing superficial and mid dermis with moderate mixed inflammatory infiltrate composed of lymphocytes, plasma cells (arrow), and neutrophils (arrow), (H&E ×400).
Our patient with non-small cell carcinoma of the lung on treatment with gefitinib presented with pustular eruption on the scalp, followed by scarring alopecia. Trichoscopy showed milky red areas with loss of hair follicles, and scalp biopsy showed mixed infiltrate of lymphocytes, plasma cells and neutrophils with absent hair follicles in the dermis, all suggestive of EPD. Therefore, we made a diagnosis of gefitinib-induced EPD with scarring alopecia.
DISCUSSION
EGFRs are present on the follicular epithelium, and gefitinib, an EGFR inhibitor, interferes with the growth of the follicular epithelium, thereby eliciting a mixed inflammatory infiltrate of neutrophils, lymphocytes, eosinophils, and plasma cells, which damages the follicular epithelium, leading to both non-scarring and scarring alopecia.[3,4] Moreover, the three phases of the hair cycle are controlled by EGFR and it functions as a switch at the beginning and the end of the anagen phase. Therefore, blocking EGFR by drugs such as gefitinib leads to alopecia.[4] Scarring alopecia is very rare. Review of literature has reported that cutaneous manifestations are not absolute indications for stopping gefitinib, unless the scarring alopecia is progressive. Erosive pustular lesions on the scalp can also be caused by pyoderma, pediculosis, and seborrhoea. Scaring alopecia can be confused with folliculitis decalvans. The S. aureus culture in our case was due to secondary infection, and even after treatment with cloxacillin, new papules and sterile pustules developed. Hence, the progression of the lesions should be attributed to gefitinib. Folliculitis decalvans has definite clinical features with clinching biopsy findings. Trichoscopy may contribute to the diagnosis of EPD as in our case, the main findings reported in the literature being milky red areas, white patches, the absence of hair follicles, tapered hairs, and hair shaft abnormalities.[5] EPD leading to scarring alopecia is very rare. Moreover, a brief review is given in Table 1.[6-9] There are no specific treatment modalities for EPD other than controlling secondary infection and stopping gefitinib. We are reporting a rare case of gefitinib-induced EPD leading to scarring alopecia, and this should be kept in mind before initiating EGFR inhibitors.
| Author | Age/sex | Underlying malignancy | Site | Treatment | Culture for bacteria | Clinical endpoint |
|---|---|---|---|---|---|---|
| Graves et al.[3] | 65/F | NSCLC | Frontal/Parietal | Discontinued GTB | Not done | Alopecia improved; residual scarring |
| Fukui et al.[4] | 57/F | NSCLC | Frontal/Parietal/Temporal | Discontinued GTB Oral antibiotics and topical steroid |
Positive | Alopecia improved; residual scarring |
| Toda et al.[5] | 69/F | NSCLC | Parietal | Discontinued GTB Oral steroids and antibiotic |
Not done | Alopecia improved; residual scarring |
| Wu et al.[6] | 69/F | LC | Frontal/Parietal | Discontinued GTB Topical steroid |
Not done | Alopecia improved; residual scarring |
| Donovan et al.[7] |
70/F | NSCLC | Frontal/Parietal | Discontinued GTB | Not done | Unknown |
| Lin et al.[8] | 60/F | NSCLC | Parieto/Temporal | Not described | Not done | Not described |
| Nazzaro et al.[9] | 84/M | NSCLC | Frontal/Parietal/Temporal | Discontinued GTB Topical steroid and salicylic acid |
Negative | Alopecia improved; residual scarring |
| Present case | 65/F | NSCLC | Frontal/Parietal | Discontinued GTB Cloxacillin, mupirocin and Clobetasol lotion |
Positive | Alopecia improved; residual scarring |
GTB: Gefitinib, NSCLC: Non-small cell cancer of lung, LC: Lung cancer, M: Male, F: Female
CONCLUSION
EGFR antagonists interfere with normal hair growth pattern and may lead to both scarring and non-scaring alopecia. This should be kept in mind before starting these agents.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
Dr. Pradeep S. Nair is on the editorial board of the Journal.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The author(s) confirms that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Status of epidermal growth factor receptor antagonists in the biology and treatment of cancer. J Clin Oncol. 2003;21:2787-99.
- [CrossRef] [PubMed] [Google Scholar]
- Dermatologic side effects associated with the epidermal growth factor receptor inhibitors. J Am Acad Dermatol. 2006;55:657-70.
- [CrossRef] [PubMed] [Google Scholar]
- Nonscarring inflammatory alopecia associated with the epidermal growth factor receptor inhibitor gefitinib. J Am Acad Dermatol. 2006;55:349-53.
- [CrossRef] [PubMed] [Google Scholar]
- Trichoscopic findings of erosive pustular dermatosis of the scalp associated with gefitinib. Case Rep Dermatol. 2017;9:44-9.
- [CrossRef] [PubMed] [Google Scholar]
- Erosive pustular dermatosis of the scalp-like eruption due to gefitinib: Case report and review of the literature of alopecia associated with EGFR inhibitors. Dermatology. 2012;225:18-21.
- [CrossRef] [PubMed] [Google Scholar]
- Erosive pustular dermatosis of the scalp after gefitinib and radiotherapy for brain metastases secondary to lung cancer. Clin Exp Dermatol. 2008;33:106-7.
- [Google Scholar]
- Scarring alopecia associated with use of the epidermal growth factor receptor inhibitor gefitinib. Arch Dermatol. 2008;144:1524-5.
- [CrossRef] [PubMed] [Google Scholar]
- Rare cutaneous side-effect of gefitinib masquerading as superficial dermatophytosis. Clin Exp Dermatol. 2009;34:528-30.
- [CrossRef] [PubMed] [Google Scholar]
- Erosive pustular dermatosis of the scalp induced by gefitinib: Case and review of the literature. Dermatol Online J. 2021;27:1-4.
- [CrossRef] [Google Scholar]






