Translate this page into:
From sweet syndrome to pemphigus foliaceus: An unusual transition
*Corresponding author: Theresa S. Kandathil, Department of Dermatology Venereology and Leprosy, Dr. Somervell Memorial CSI Medical College Hospital, Thiruvananthapuram, Kerala, India. theresa96sk@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Kandathil TS, Maneek KF, Samson JF, Rema Yesudasan RD. From sweet syndrome to pemphigus foliaceus: An unusual transition. J Skin Sex Transm Dis. doi: 10.25259/JSSTD_10_2025
Abstract
A 70-year-old lady presented with acute onset of tender erythematous papules and plaques associated with fever and severe fatigue. Histopathology showed predominant neutrophilic dermal infiltration, and a diagnosis of sweet syndrome was made. The patient started on oral doxycycline and topical steroids, and the lesions started resolving. Two months later, the patient presented with altered morphology of lesions, simulating pemphigus foliaceus, and it was confirmed with a Tzanck smear, histopathology, and direct immunofluorescence. The patient started on dexamethasone-cyclophosphamide pulse therapy and has responded well. It is worth considering whether the first appearance, which resembles sweet syndrome, is a precursor of pemphigus foliaceus or an aberrant presentation of pemphigus itself. The occurrence of both conditions in the same individual in such a short period of time makes this a unique case.
Keywords
Autoimmune blistering disease
Neutrophilic dermatosis
Paraneoplastic dermatoses
Pemphigus foliaceus
Sweet syndrome
INTRODUCTION
Sweet syndrome, or acute febrile neutrophilic dermatosis, was first described in 1964 by Robert Douglas Sweet. The four cardinal features of the disease are fever, neutrophilia, painful plaques distributed asymmetrically across the face, neck, and upper extremities, and dense dermal infiltrates with mature neutrophil polymorphs.[1] The disease may resolve spontaneously, but most cases will require corticosteroids for an adequate response. Very rarely, several other steroid-sparing agents may be necessary.[2]
Pemphigus foliaceus is a superficial variant of pemphigus. It is characterized by the presence of circulating antibodies against desmoglein-1, which destroy the adhesion between epidermal cells, thereby producing blisters.[3] The onset is insidious, with crusted erosions on an erythematous base, resembling “cornflake.” Lesions predominantly involve seborrheic areas: Scalp, face, chest, and upper back, sparing mucosa. Systemic symptoms are usually absent.[4] A more aggressive and longer duration of treatment as compared to sweet syndrome is necessary for pemphigus patients.
This case report discusses a unique clinical scenario involving a 70-year-old female who presented initially with features suggestive of sweet syndrome but subsequently developed lesions of pemphigus foliaceus within a short period of 3 months. It prompts consideration of whether sweet syndrome could be a precursor of or a rare, atypical presentation of pemphigus foliaceus.
CASE REPORT
A 70-year-old lady came to the emergency department of our tertiary care center with a history of sudden-onset skin rash with fever, malaise, and reduced appetite for 4 days duration. She also complained of nausea and vomiting of 1-day duration. Two weeks before the present episode, there was a history of upper respiratory tract infection. She was on treatment for systemic hypertension and is also on thyroid hormone replacement following thyroidectomy. No change in regular medications or new drugs started before the onset of symptoms. Lesions were multiple discrete erythematous papules and plaques with burning sensation and minimal itching, predominantly involving the face and neck [Figure 1a]. There was no ocular, oral, or genital involvement and no lymphadenopathy. There was no associated arthralgia. With the acute presentation of painful erythematous papules and plaques associated with fever, a diagnosis of sweet syndrome was considered. Investigations to rule out infectious and malignant causes were done, all of which came negative, except for an elevated erythrocyte sedimentation rate. A biopsy specimen was obtained from an erythematous plaque on the face, which revealed epidermal spongiosis and dense perivascular infiltrates of neutrophils in the dermis [Figure 1b]. The clinical presentation and histopathological findings pointed to a diagnosis of acute febrile neutrophilic dermatosis, i.e., sweet syndrome. The patient was initially treated with topical steroids. Considering the risk factors related to immunosuppressive treatments in older adults, doxycycline was chosen as a safer treatment choice. The patient began to show improvement after starting oral doxycycline at a dose of 100 mg twice a day.

- (a): Initial clinical presentation; (b): Histopathology showing epidermal spongiosis and dense perivascular infiltrates of neutrophils in the dermis (Hematoxylin and eosin, ×10).
After two months, she presented with gastritis, but this time the rashes had an altered morphology from the previous ones. They appeared as crusted erosions on the face (predominantly on the cheeks), scalp, and a few on the trunk, associated with mild itching [Figure 2a]. As the clinical features were very typical, a provisional diagnosis of pemphigus foliaceus was made and investigations to confirm the same were done. Tzanck smear showed few acantholytic cells, and a repeat skin biopsy sample was taken from the lesion on the face, in which sub-corneal blister formation with acantholytic cells and few neutrophils were found [Figure 2b]. Direct immunofluorescence (DIF) showed intercellular staining of the epidermis with immunoglobulin G (IgG) and C3, which confirmed the diagnosis of pemphigus foliaceus. She was started on dexamethasone-cyclophosphamide pulse (DCP) therapy and showed remarkable improvement in the skin lesions. The patient completed phases 1 and 2 of DCP and is currently in phase 3 and is doing well.
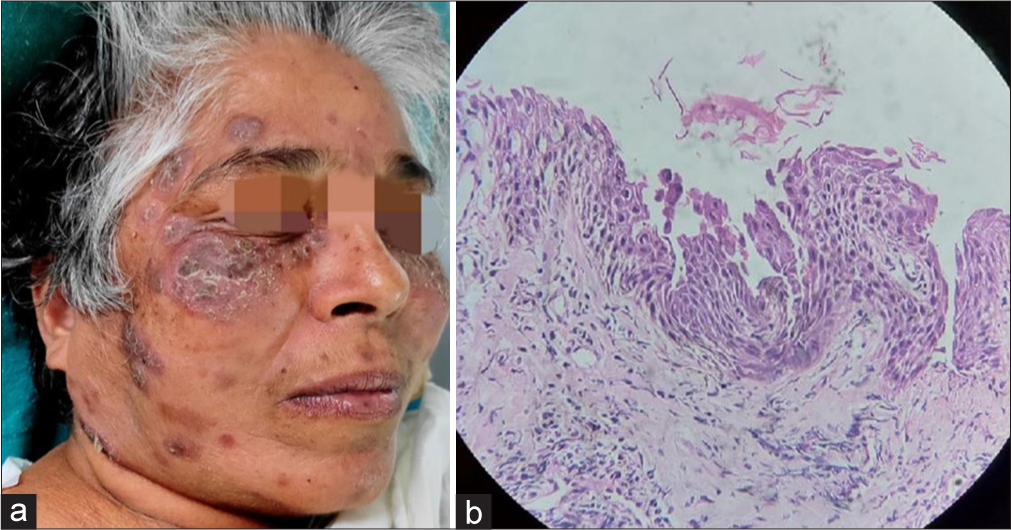
- (a): Clinical picture after three months; (b): Histopathology showing sub-corneal blister formation with acantholytic cells and few neutrophils (Hematoxylin and eosin, ×40).
DISCUSSION
Sweet syndrome refers to an acute febrile neutrophilic dermatosis with painful, oedematous, erythematous, well-circumscribed nodules or plaques that generally appear on the head, neck, and upper limbs.[5] It has a female preponderance, with an average age of onset between 30 and 60 years. Other than malignancies, associations include a variety of inflammatory and autoimmune conditions, including inflammatory bowel illness (ulcerative colitis or Crohn’s disease), rheumatoid arthritis, systemic lupus erythematosus, Sjogren syndrome, Hashimoto thyroiditis, Behcet disease, and dermatomyositis.[2] Drugs known to induce the condition include colony-stimulating factors, antibiotics, antiepileptics, highly active antiretroviral therapy, antihypertensives, chemotherapeutic agents, contraceptives, diuretics, nonsteroidal anti-inflammatory drugs, and retinoids.[6] Any external or internal stimulus alters cell signaling and cytokine synthesis, triggering an increase in neutrophil production and migration to the skin. The typical histology consists of a diffuse, dense neutrophilic infiltrate in the reticular dermis. Papillary dermal edema is common without any vasculitis features.[2]
The initial presentation of our patient satisfied the major criterion for diagnosing sweet syndrome, i.e., the tender erythematous papule and plaques with predominant neutrophilic dermal infiltrate. The absence of any blisters or crusted erosions, along with the development of other constitutional symptoms, contributed to the diagnosis. Paraneoplastic and drug-induced sweet syndrome were ruled out with adequate history-taking, meticulous clinical examination, and relevant investigations. Post-infectious sweet syndrome has been described 1–3 weeks after upper respiratory and gastrointestinal infections.[2] In our case, the patient had an upper respiratory tract infection of two-week duration, which we assumed to have triggered the event. The development of crusted erosions over the same sites after two months was in striking contrast to the preliminary/prior lesions. Acantholytic cells and the intercellular IgG and C3 deposits in the DIF helped us arrive at our diagnosis as pemphigus foliaceus.
Pemphigus foliaceus is an autoimmune blistering disorder that is insidious in onset with scattered, crusted lesions involving the seborrheic areas: Scalp, face, chest, and upper back. Patients with pemphigus foliaceus have autoantibodies directed against desmoglein-1, which destroys the integrity of epidermal intercellular adhesion and leads to the formation of crusted erosions and blisters. Desmoglein-1 is expressed more in the skin of the upper trunk but only weakly in mucosae, which explains the sparing of mucosa. It predominantly involves the elderly age group and may have a chronic relapsing course. It is less common and less severe than pemphigus vulgaris.[3] Histology shows an upper epidermal subcorneal blister containing acantholytic keratinocytes, neutrophils, and fibrin. Systemic steroids (as pulses) along with steroid-sparing adjuvants are the treatment of choice. Immunosuppressants such as rituximab, cyclophosphamide, methotrexate, mycophenolate, or azathioprine are other alternatives. Intravenous immunoglobulin, immunoadsorption, or plasmapheresis have shown promising improvement in the prognosis.[6]
Typical clinical and histological features may not be present in the early stages of pemphigus foliaceus, and this may lead to misdiagnosis in some instances. Pemphigus foliaceus can resemble seborrheic dermatitis or impetigo, and the rare, documented presentations include eruptive seborrheic keratoses, dermatitis herpetiformis, or acanthosis nigricans-like lesions.[7,8] It is to ponder whether the first presentation simulating sweet syndrome can be considered either as a harbinger of pemphigus foliaceus or as an atypical presentation of pemphigus foliaceus. One case report describing a 58-year-old female patient with newly diagnosed pemphigus vulgaris who later presented with erythematous plaques and leucocytosis, highly suggestive of sweet syndrome is reported earlier.[9] However, the vice versa, i.e., evidence of sweet syndrome preceding pemphigus, is new. Any other association between the two diseases is not yet established.
CONCLUSION
Sweet syndrome and pemphigus foliaceus are fundamentally distinct conditions. Their differences in etiology, pathogenesis, clinical presentation, and treatment approaches clearly establish them as separate and unrelated disorders. We report a case of sweet syndrome preceding pemphigus foliaceus within a duration of three months. The unusual sequence of events in this case raises intriguing questions about the interplay between autoimmune and inflammatory processes. It also underscores the need for clinicians to maintain a high index of suspicion and consider a broader differential diagnosis when clinical and histological features evolve over time.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship: Nil.
References
- An acute febrile neutrophilic dermatosis. Br J Dermatol. 1964;76:349-56.
- [CrossRef] [PubMed] [Google Scholar]
- Neutrophilic dermatoses: A review of current treatment options. Am J Clin Dermatol. 2009;10:301-12.
- [CrossRef] [PubMed] [Google Scholar]
- Regional variation in the expression of pemphigus foliaceus, pemphigus erythematosus, and pemphigus vulgaris antigens in human skin. J Invest Dermatol. 1991;96:159-61.
- [CrossRef] [PubMed] [Google Scholar]
- An updated review of pemphigus diseases. Medicina (Kaunas). 2021;57:1080.
- [CrossRef] [PubMed] [Google Scholar]
- Paraneoplastic dermatoses: A brief general review and an extensive analysis of paraneoplastic pemphigus and paraneoplastic dermatomyositis. Int J Mol Sci. 2020;21:2178.
- [CrossRef] [PubMed] [Google Scholar]
- Rook's textbook of dermatology Vol 4. (9th ed). Chichester, West Sussex, Hoboken, NJ: John Wiley and Sons Inc; 2016. p. :50.1-9.
- [CrossRef] [Google Scholar]
- Pemphigus foliaceus resembling eruptive seborrheic keratoses. Arch Dermatol. 1980;116:815-6.
- [CrossRef] [PubMed] [Google Scholar]
- Pemphigus foliaceus associated with acanthosis nigricans-like lesions and hepatocellular carcinoma. Int J Dermatol. 1989;28:462-3.
- [CrossRef] [PubMed] [Google Scholar]
- Sweet syndrome and pemphigus vulgaris. J Cutan Med Surg. 2012;16:128-30.
- [CrossRef] [PubMed] [Google Scholar]







