Translate this page into:
Hydroxychloroquine
*Corresponding author: Sandhya George, Department of Dermatology and Venereology, Government Medical College, Manjeri, Kerala, India. drsandhyageorge@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Sindhu CB, George S, Sankar A, Stephen V. Hydroxychloroquine. J Skin Sex Transm Dis 2021;3(1):33-9.
Abstract
Hydroxychloroquine (HCQ) and its related drug, chloroquine, have been under use for malaria for the past 75 years. Its use for malaria, rheumatoid arthritis and systemic lupus erythematosus are Food and Drug Administration (FDA) approved. These drugs have immunomodulatory and antiviral actions. More and more indications for this drug are being explored. These drugs are still under study as possible treatments for coronavirus disease 2019 but at present FDA has revoked its emergency use authorization for these two drugs. However, in many other indications, HCQ is a valuable drug but monitoring for adverse effects is mandatory.
Keywords
Hydroxychloroquine
Coronavirus disease 2019
Indications
Monitoring
HISTORY
In 1638, when the wife of the Viceroy of Peru countess Cinchona acquired malaria while living in the new world, she was treated by an herbalist with bark of a tree, and she had dramatic response.[1] The tree was eventually named after the countess as “Cinchona tree.” When the Viceroy returned to Spain, he took large supplies of the powder, made from the bark of this tree with him. At that time its supply was controlled by Church and hence called “Jesuits Powder” and the tree bark called “Jesuit’s bark.”[1] It took nearly two centuries for the active substance “quinine” to be isolated from the tree bark in 1820. Quinine replaced the crude preparation and continued to be the major antimalarial drug till 1942.[1]
By 1940 quinine derivative chloroquine was recognized for its antimalarial property and was found useful among troops fighting in the Pacific during world war 2, but this drug was noted to have significant toxicities.[2] In 1945, a modification of this compound by hydroxylation led to the development of hydroxychloroquine (HCQ).[3] HCQ was found to be less toxic and remains in use without change to this day.
Cinchona is a genus of flowering plant in the family Rubiaceae. HCQ is a 4-aminoquinoline with immunosuppressive, antiautophagy, and antimalarial activities.[4] HCQ was initially developed as an antimalarial for malaria patients who were sensitive to chloroquine. HCQ sulfate tablet contains 200 mg HCQ sulfate equivalent to155 mg base and is used for oral administration.[4]
Molecular formula: C18H26ClN3O [Figure 1][4]
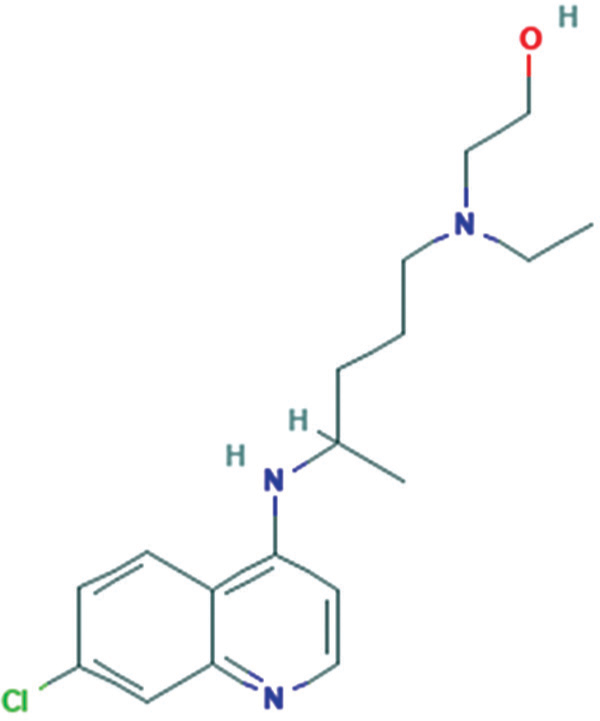
- Molecular structure of hydroxychloroquine
ABSORBTION, METABOLISM AND EXCRETION
HCQ is 67%–74% bioavailable. A 200 mg oral dose of HCQ has a half-life of 22.4 days in blood and 123.5 days in plasma. HCQ is N-dealkylated by cytochrome CYP3A4 to the active metabolite desethyl HCQ, as well as the inactive metabolites desethyl chloroquine and bidesethylchloroquine. About 40%–50% of HCQ is excreted renally, while only 16%–21% of a dose is excreted in the urine as unchanged drug. 5% of a dose is sloughed off in skin and 24%–25% is eliminated through the feces.[4]
Mechanism of action
The exact mechanism by which antimalarials act in various diseases is not fully understood, but there is strong evidence that they have an immunomodulatory and antithrombotic effect in chronic inflammatory conditions. The proposed mechanisms to explain the different effects are[5]
Alkalinization of lysosomes and other intracellular acid compartments with interference in phagocytosis. The increase of intracellular pH causes a selective change in the presentation of proper antigens
Blockage of T-cell response and reduction of pro-inflammatory cytokine production, including INF-γ, TNF, IL-1, and IL-6
Blockage of toll-like receptors 7 and 9, especially in plasmacytoid dendritic cell with inhibition of INF-α, which plays an important role in the pathophysiology of SLE
The cyclic GMP-AMP synthase-stimulator of interferon genes (cGAS-STING) pathway has an important role in the immune system. This signaling is inhibited by antimalarials
Inhibition of phospholipase A2 activity
Stimulation of nitric oxide production by endothelial cells with antiproliferative effect
Antithrombotic effect: This is due to inhibition of platelet aggregation in a dose-dependent manner, decreased production of arachidonic acid by activated platelets and action on antiphospholipid antibodies
Action on glucose metabolism and lipid profile as a nonimmunomodulatory mechanism
Antiviral effect: Chloroquine/HCQ may inhibit entry, replication, and spread of several viruses by different mechanisms some of which have already been demonstrated or proposed for Sars-Cov-2 infection. Possible mechanisms are by increasing endosomal pH, by immune-modulation, and by interference with terminal glycosylation of the cellular receptor ACE2 (Angiotensin-converting enzyme).[6] It was also recently described that chloroquine might inhibit quinone reductase-2, an enzyme involved in sialic acid biosynthesis.[7]
INDICATIONS
US-Food and Drug Administration (FDA) approved indications
FDA approved indications include malaria, rheumatoid arthritis (RA), and lupus erythematous (LE).[8]
Malaria
HCQ is indicated for the treatment of uncomplicated malaria due to Plasmodium falciparum, Plasmodium malariae,Plasmodium ovale, and Plasmodium vivax. It is also indicated for the prophylaxis of malaria in geographic areas where chloroquine resistance is not reported.[9]
RA
HCQ is approved for the treatment of acute or chronic RA.[10] The initial dosage is 400–600 mg/day; depending on patient’s body weight. If a good clinical response is seen over a period of 4–12 weeks, the dosage can be reduced by 50% and continued at 200–400 mg/day. The risk of retinopathy is greater when this dosage is exceeded.
Lupus erythematosus
HCQ is recommended for all patients with LE in discoid LE (DLE), sub-acute LE (SCLE), lupus panniculitis (profundus), and SLE.[8,11] In DLE, antimalarials are used in patients who fail to respond well to topical measures such as sunscreens and topical steroids. About 60%–70% of DLE respond to HCQ. Hypertrophic/verrucous LE responds less to antimalarials. In a randomized double blind study that lasted 8 weeks for cutaneous LE, 400 mg HCQ was as effective as 50 mg acitretin (response rate of about 50%), and showed fewer side effects.[12] In SCLE, the reported response rate is 50%–75 %.[8]
The drug can be given during pregnancy.[13] Patients who discontinue HCQ after being on it for less than 1 year are at greater risk for flares compared with those who take HCQ for longer than 1 year.[14] There is evidence for multiple beneficial effects of HCQ in SLE, not only in skin inflammation but also in arthritis, pleuritis, pericarditis, and lethargy.[14]
HCQ is given at a dose of <6.5 mg/kg/day based on ideal body weight. Usual dose given is 200–400 mg/day scheduled as single dose or two divided doses.[8]
Antimalarials are slow acting and at least 3–6 months of treatment are required to see results.[15] HCQ monotherapy is preferred over chloroquine given its improved safety profile, in particular with regard to retinal toxicity.[16] HCQ usage is independently associated with a reduced risk of damage accrual (cumulative damage) in SLE patients who had not yet accrued damage at the time of initiation of the treatment.[17]
OFF-LABEL USES
Coronavirus disease 2019 (COVID-19)
FDA revoked the emergency use authorization that allowed the use of HCQ in the treatment of certain hospitalized patients with COVID-19 infection.[18] This was based on the lack of evidence for its benefit in COVID-19 management. At the same time there were reports of large increase in adverse events (especially cardiac) including patient deaths, due to these drugs. However, in India, it is still approved in the treatment of COVID-19 infection (400 mg twice daily on 1st day followed by 200mg twice a day for 4 days, after assessment of ECG.[19] The National Task Force for COVID – constituted by the Indian Council for Medical Research on March 22, 2020 – recommended the use of HCQ for prophylaxis of SARS-CoV-2 infection for healthcare workers and household contacts of COVID-19 patients.[20] The decision was based on available preclinical and clinical data and anecdotal reports.
Other viral diseases
HCQ reduces Zika viral transmission to fetus, HCQ significantly decreases Zika virus infection in placental cells, possibly by inhibition of NS2B- NS3 protease which plays an essential role in hydrolysis and maturation of the flavivirus polyprotein.[21] It has got some effect against dengue virus also.[22]
Porphyria cutanea tarda
Antimalarials are not the first line therapy in porphyria cutanea tarda. For patients who are anemic or who fail to respond to phlebotomy, antimalarials may be considered.[23] Low dose antimalarials (chloroquine 125 mg 2 times weekly or HCQ 100 mg 3 times weekly) that is roughly 10% of usual dose is used in this condition. This should not be used if there is renal failure.
Other photodermatoses
Polymorphic light eruption, solar urticaria, dermatomyositis (controversial), and reticular erythematous mucinosis show response to HCQ.[8, 24] HCQ was found to be significantly more effective than chloroquine in the treatment of polymorphic light eruption with little risk of ocular toxicity.[25]
Granulomatous dermatoses
In sarcoidosis, HCQ 200–400 mg is effective in patients with dermatologic involvement, joint manifestations, and hypercalcemia.[26] Other conditions in which it has been tried are granuloma annulare, generalized necrobiosis lipoidica, necrobiotic xanthogranuloma, interstitial granulomatous dermatitis, and annular elastolytic giant cell granuloma.[8,27]
Panniculitis
HCQ is found to be effective for nodular panniculitis (Weber Christian Disease), recurrent erythema nodosum and lupus panniculitis in the usual dose.[28]
Other conditions
Morphea, urticarial vasculitis, cutaneous or systemic vasculitis, graft versus host disease, livedoid vasculopathy, perniosis, follicular mucinosis, psoriatic arthritis, benign lymphocytic infiltrate like lymphocytoma cutis, and Jessner’s lymphocytic infiltrate are other conditions where HCQ is effective.[29-36]
DRUG INTERACTIONS
Table 1 lists drug interactions of HCQ.[8,37] The most significant drug interactions of concern are those between different antimalarials. Chloroquine and HCQ should not be administered concurrently because of the additive retinotoxic potential. The combination with primaquine used for malaria prophylaxis can induce hemolysis in glucose-6-phosphate dehydrogenase (G6PD)-deficient patient.[8]
| Drugs | Interactions |
|---|---|
| Chloroquine | Increased risk of retinal toxicity |
| Primaquine | Hemolytic anemia |
| Mefloquine (8-aminoquinolines) | QT prolongation risk with potential for torsades de pointes |
| Tamoxifen | Retinal toxicity |
| Antipsychotic agents pimozide | QT prolongation risk with potential for torsades de pointes |
| Macrolides-erythromycin, clarithromycin | QT prolongation risk with potential for torsades de pointes |
| Bupropion, mefloquine, tramadol, propafenone | Increase the risk of seizures |
| Dapsone | Hemolysis |
| Cimetidine | Decreases clearance of antimalarials with resultant increase in serum levels and potential toxicity |
| Inotropic agents - digoxin, antiarrhythmics, propafenone | Clearance of digoxin is decreased; hence, toxicity is increased. Digoxin decreases clearance of both antimalarials |
| Cyclosporine | Synergistic action when combined, hence, toxicity of both may increase |
| Local anesthetics - benzocaine, prilocaine, tetracaine | Combination may increase risk of hemolysis, methemoglobinemia (low risk unless used on large surface area) |
| Botulinum toxin | Decrease activity of botulinum toxin |
| Antacids-aluminum, calcium, magnesium, proton pump inhibitors, H2 blockers | May inhibit absorption of antimalarials |
| Miscellaneous - gemfibrozil, clopidogrel, verapamil, diltiazem, azoles, ciprofloxacin | Increase the level of HCQ |
HCQ increases cyclosporine and digoxin levels, which is attributed to inhibition of P-glycoprotein (P-gp) activity. The P-gp transport system is an efflux transporter found most notably in gut luminal and blood-brain barrier endothelial cells. Chloroquine and HCQ are inhibitors of this transporter/pump.[37]
The cytochrome (CYP) P450 system is the main family of enzymes responsible for Phase I oxidative metabolism of numerous drugs. Antimalarials undergo metabolism by several enzymes of this system. HCQ is a substrate of CYP2C8, CYP3A4/5, and CYP2D6. Elevation of metoprolol levels is through CYP2D6 inhibition.
Coadministration of inhibitors of CYP2C8 (gemfibrozil and clopidogrel) and inhibitors of CYP 3A4/5 (verapamil, diltiazem, azole anti-fungal agents, most macrolide antibiotics, and ciprofloxacin, among others) may potentially raise the blood levels of chloroquine and HCQ.[37]
Adverse effects
Common side effects are the following:[8]
Ocular—reversible
Corneal deposition — halos, blurred vision, and photophobia (especially chloroquine)
Loss of accommodation (especially chloroquine)
Premaculopathy — usually no visual change; typically, retinal pigment deposition, paracentral, and pericentral scotoma.
Ocular — irreversible
True retinopathy — “bull’s eye” pigment deposition, central scotoma, and visual acuity changes (risk greatest with chloroquine; and no risk with quinacrine).
Hematologic
Rarely agranulocytosis or pancytopenia
Hemolysis in G6PD deficient patients (primarily a risk with primaquine and other 8-aminoquinolines).
Cardiovascular
Cardiovascular toxicity include long QT syndrome, torsade de pointes, cardiomyopathy and sudden cardiac deaths.[38] Of all the adverse drug reactions, cardiac toxicity has gained more importance recently. These drugs can prolong the QT interval. Drug-induced QT prolongation per se is asymptomatic; however, it can lead to Torsade de Pointes, which is a potentially lethal polymorphic ventricular tachycardia. These patients present with syncope with or without convulsions, and sudden cardiac death.[38]
Gastrointestinal
Nausea, vomiting, and diarrhea (10% of patients on chloroquine have intolerable gastrointestinal effects)
changes in liver function test— transaminase elevations (uncommon).
Neuromuscular
Irritability, nervousness, and mood swings
Psychosis • Headache • Seizures (rare)
Vertigo, tinnitus, and nystagmus
Skeletal muscle weakness
Neuropathy.
Myopathy and, to a lesser extent, neuropathy are well-documented complications of therapy with antimalarial agents.[38] Antimalarial myopathy is a poorly recognized entity and should be suspected if patients show elevated muscle enzymes. This should be confirmed by muscle biopsy.
Cutaneous
Bluish-gray hyperpigmentation (especially shins, face, and palate)
Bleaching of hair roots
Minor hypersensitivity reactions — morbilliform, lichenoid, and eczematous
More severe hypersensitivity reactions — urticaria and erythroderma
Psoriasis — induction or exacerbation
Nails — transverse pigment bands.
In the scenario of COVID-19, a review on adverse effects of HCQ and chloroquine was conducted. Key findings were
The majority of the cases (69%) involved males with a median age in the early 60 s[39]
Of the 385 cases reporting adverse effects of HCQ or chloroquine in the setting of COVID-19, 377 cases reported use for treatment and eight cases reported use for prophylaxis
Eleven cases reported both cardiac and non-cardiac adverse effects. Of all serious adverse events (cardiac and non-cardiac), QT prolongation was the most commonly reported adverse event for both HCQ and chloroquine
Serious cardiac adverse event was seen in 109 patients: 92 (84%) patients reported concomitant use of at least one other medication that prolongs the QT interval. 75 (69%) reported concomitant use of azithromycin. 80 (73%) reported QT prolongation, 4 (3.7%) reported torsades de pointes, 14 (13%) reported ventricular arrhythmia, ventricular tachycardia, or ventricular fibrillation, and 25 (23%) had a fatal outcome. 22 out of the 25, who had a fatal outcome reported the use of a concomitant QT-prolonging medication.
Non-cardiac adverse effects were reported in 113 out of 385 cases. These included hepatitis/increased liver enzymes/ hyperbilirubinemia in 59% cases, acute kidney injury in 5% cases and methemoglobinemia in 4 patients, the latter two are currently not labeled as side effects of HCQ.
MONITORING GUIDELINES
Ophthalmological monitoring
Chloroquine and HCQ to a lesser extent have been known to produce retinopathy as well as vortex keratopathy or cornea verticillate.[40] While keratopathy is reversible and does not affect visual acuity, retinopathy is irreversible and can lead to visual impairment. Moreover, it can progress even after stopping treatment, more so with severity of the retinopathy. Hence, it is important to have effective screening techniques to detect early retinal changes before visual function is affected.[40]
Risk factors for ocular toxicity
Most significant risk factors are daily dose of >5 mg/day/real weight for HCQ and 2.3 mg/day/real weight for chloroquine and duration of therapy of >5 years. Patients on 5.0 mg/kg have <1% risk in the first 5 years of therapy and less than 2% up to 10 years with the risk increasing sharply to approximately 20% after 20 years.[40] Patients on HCQ for more than 5 years showed an overall prevalence of toxicity of 7.5% when tested using 10–2 visual fields or spectral-domain optical coherence tomography (SD OCT) before there were any visible signs on fundus examination.[40]
Other risk factors are concomitant renal disease with subnormal glomerular filtration rate, concomitant use of tamoxifen and pre-existing macular disease which may affect screening and susceptibility to the HCQ/chloroquine.[41]
Screening schedule
It is the responsibility of the prescribing physician to refer patients eligible for monitoring to the ophthalmologist specifying the key clinical details relevant to monitoring for retinal toxicity. This will help in determining the risk of toxicity and in interpreting the test results.[42]
According to the 2016 revised American Academy of Ophthalmology recommendations, a baseline fundus examination should be done within 1 year of starting therapy to rule out a pre-existing maculopathy and to establish a record of the fundus appearance and functional status. Baseline visual fields and SD OCT are usually done, though it is not mandatory to obtain them unless abnormalities are present. Annual screening is started after 5 years of exposure if there are no risk factors. Screening should begin sooner and more frequently for patients with major risk factors.[41]
The recommended screening tests are as follows:[42]
Automated visual fields – Central 24–2/30–2 fields for Asian patients and Central 10–2 for non-Asian patients should be done as Asians were found to have extramacular pattern of damage unlike non-Asians with parafoveal damage. This being a subjective test with variability, it may have to be repeated
SD-OCT- is an objective test which is highly specific and sensitive. Localized thinning of photoreceptor layers in the parafoveal or extramacular areas is a strong indicator of toxicity
-
Other objective tests (done as required).
Fundus autofluorescence (FAF) – early toxicity is seen as ring of increased autofluorescence and later as decreased autofluorescence once retinal pigment epithelium is lost.
Multifocal electroretinogram (ERG) – Can provide objective confirmation of visual field loss by showing localized ERG depression.
Unless toxic changes are advanced and obvious, at least one objective test should confirm subjective findings before toxicity is diagnosed. This is to avoid unnecessary stoppage of this valuable medication.[40] Once definite toxicity is diagnosed, decision to stop therapy should be made in conjunction with the patient and the treating physician. Risk of further visual loss depending on severity should be explained to the patient.
Monitoring of blood parameters
Complete blood count
G6PD screening (only needed in selected clinical settings)
Automated chemistry profile (emphasis on liver function tests)
Random or 24-hour urinary porphyrin screening (alternatively may order serum porphyrins) – only if porphyria is clinically suspected.
Follow-up
CBC (monthly for 3 months, then every 4–6 months)
Automated chemistry profile (after 1 month, after 3 months, and then every 4–6 months)
HCQ blood levels may be useful to predict response and identify patients who are noncompliant.
CONCLUSION
HCQ is a drug which is useful in many conditions. Although its use is controversial in COVID-19, it is widely used in other conditions. As it has got drug interactions and toxicity especially after prolonged use, proper monitoring is mandatory. Hence, patients should be informed about and prescribing physicians should be aware of risk of toxicity, proper dose levels, and the importance of regular annual screening.
Declaration of patient consent
Not required as there are no patients in this article.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Cinchona. In: Encyclopaedia Britannica Vol 5. (9th ed.). New York: Charles Scribner's Sons; 1878. p. :780-2.
- [Google Scholar]
- Saving Lives, Buying Time: Economics of Malaria Drugs in an Age of Resistance Washington, DC: National Academies Press; 2004. p. :126-8.
- [Google Scholar]
- Hydroxychloroquine: An old drug with new relevance. Cleve Clin J Med. 2018;85:459-67.
- [CrossRef] [PubMed] [Google Scholar]
- PubChem Compound Summary for CID 3652 Hydroxychloroquine Bethesda, MD: National Center for Biotechnology Information; 2020.
- [Google Scholar]
- Revisiting hydroxychloroquine and chloroquine for patients with chronic immunity-mediated inflammatory rheumatic diseases. Adv Rheumatol. 2020;60:32.
- [CrossRef] [PubMed] [Google Scholar]
- Antiviral mechanisms of candidate chemical medicines and traditional Chinese medicines for SARS-CoV-2 infection. Virus Res. 2020;286:198073.
- [CrossRef] [PubMed] [Google Scholar]
- Hydroxychloroquine and covid-19. Postgrad Med J. 2020;96:550-5.
- [CrossRef] [PubMed] [Google Scholar]
- Antimalarial agents. Comprehensive Dermatologic Drug Therapy 2020:234-44.
- [CrossRef] [Google Scholar]
- Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2019 [Last accessed on 2020 Aug 30]
- Rheumatoid Arthritis (RA) Medication. Medscape, Drugs and Diseases, Rheumatology 2020 Available from: http://www.medscape>drugsanddiseases>rheumatoidarthritis(ra)medication. [Last accessed on 2020 Feb 07]
- [Google Scholar]
- Case report on a patient with lupus panniculitis. Postepy Dermatol Alergol. 2015;32:59-62.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment of cutaneous lupus erythematosus with acitretin and hydroxychloroquine. Br J Dermatol. 1992;127:513-8.
- [CrossRef] [PubMed] [Google Scholar]
- Hydroxychloroquine (HCQ) in lupus pregnancy: Double-blind and placebo-controlled study. Lupus. 2001;10:401-4.
- [CrossRef] [PubMed] [Google Scholar]
- Hydroxychloroquine in dermatology and beyond: Recent update. Indian Dermatol Online J. 2020;11:453-64.
- [Google Scholar]
- Outcomes of systemic lupus erythematosus in patients who discontinue hydroxychloroquine. ACR Open Rheumatol. 2019;1:593-9.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical efficacy and side effects of antimalarials in systemic lupus erythematosus: A systematic review. Ann Rheum Dis. 2010;69:20-8.
- [CrossRef] [PubMed] [Google Scholar]
- Systemic lupus erythematosus in three ethnic groups: XVI. Association of hydroxychloroquine use with reduced risk of damage accrual. Arthritis Rheum. 2005;52:1473-80.
- [CrossRef] [PubMed] [Google Scholar]
- Coronavirus (COVID-19) Update: FDA Revokes Emergency Use Authorization for Chloroquine and Hydroxychloroquine Maryland: Food and Drug Administration; 2020.
- [Google Scholar]
- Available from: https://www.mohfw.gov.in/pdf/ClinicalManagementProtocolforCOVID19.pdf [Last accessed on 2020 Aug 27]
- Advisory on the Use of Hydroxy-Chloroquine as Prophylaxis for SARS-CoV-2 Infection. 2020. Available from: https://www.mohfw.gov.in/pdf/yontheuseofHydroxychloroquinasprophylaxisforSARSCoV2infection.pdf [Last accessed on 2020 Aug 31]
- [Google Scholar]
- Hydroxychloroquine inhibits Zika virus NS2B-NS3 protease. ACS Omega. 2018;3:18132-41.
- [CrossRef] [PubMed] [Google Scholar]
- Hydroxychloroquine-inhibited dengue virus is associated with host defense machinery. J Interferon Cytokine Res. 2015;35:143-56.
- [CrossRef] [PubMed] [Google Scholar]
- Low-dose hydroxychloroquine is as effective as phlebotomy in treatment of patients with porphyria cutanea tarda. Clin Gastroenterol Hepatol. 2012;10:1402-9.
- [CrossRef] [PubMed] [Google Scholar]
- Aminoquinoline antimalarial therapy in dermatomyositis-are we missing opportunities with respect to comorbidities and modulation of extracutaneous disease activity? Ann Transl Med. 2018;6:154.
- [CrossRef] [PubMed] [Google Scholar]
- Comparative study of efficacy and safety of hydroxychloroquine and chloroquine in polymorphic light eruption: A randomized, double-blind, multicentric study. Indian J Dermatol Venereol Leprol. 2008;74:18-22.
- [CrossRef] [PubMed] [Google Scholar]
- Hydroxychloroquine is effective therapy for control of cutaneous sarcoidal granulomas. J Am Acad Dermatol. 1990;23:487-9.
- [CrossRef] [Google Scholar]
- Beneficial effects of antimalarials in the treatment of generalized granuloma annular in children. Tunis Med. 2006;84:125-7.
- [Google Scholar]
- Update on management of connective tissue panniculitides. Dermatol Ther. 2012;25:173-82.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment of morphea with hydroxychloroquine: A retrospective review of 84 patients at Mayo Clinic 1996-2013. J Am Acad Dermatol. 2019;80:1658-63.
- [CrossRef] [PubMed] [Google Scholar]
- AB0531 the role of hydroxychloroquine in ANCA positive and negative vasculitis. Ann Rheum Dis. 2016;75:1086-7.
- [CrossRef] [Google Scholar]
- Randomized trial of hydroxychloroquine for newly diagnosed chronic graft-versus-host disease in children: A children's oncology group study. Biol Blood Marrow Transplant. 2012;18:84-91.
- [CrossRef] [PubMed] [Google Scholar]
- Livedoid vasculopathy: A review of pathogenesis and principles of management. Indian J Dermatol Venereol Leprol. 2016;82:478-88.
- [CrossRef] [PubMed] [Google Scholar]
- Successful treatment of perniosis with hydroxychloroquine. J Drugs Dermatol. 2010;9:1242-6.
- [Google Scholar]
- Treatment of so-called idiopathic follicular mucinosis with hydroxychloroquine. Br J Dermatol. 2010;163:420-3.
- [CrossRef] [PubMed] [Google Scholar]
- AB0827 hydroxychloroquine does not increase psoriasis in psoriatic arthritis: Time on drug analysis based on real life data. Ann Rheum Dis. 2015;74:1177.
- [CrossRef] [Google Scholar]
- Jessner Lymphocytic Infiltration of the Skin. 2017. Medscape. Available from : http://www.emedicine.com/derm/topic200.htm [Last accessed on 2020 Jul 31]
- [Google Scholar]
- Safety considerations with chloroquine, hydroxychloroquine and azithromycin in the management of SARS-CoV-2 infection. CMAJ. 2020;192:E450-3.
- [CrossRef] [PubMed] [Google Scholar]
- Chloroquine and hydroxychloroquine in coronavirus disease 2019 (COVID-19) Facts, fiction and the hype: A critical appraisal. Int J Antimicrob Agents. 2020;56:106101.
- [CrossRef] [PubMed] [Google Scholar]
- Office of Surveillance and Epidemiology Pharmacovigilance Memorandum Maryland: Food and Drug Administration, Center for Drug Evaluation and Research; 2020.
- [Google Scholar]
- The risk of toxic retinopathy in patients on long-term hydroxychloroquine therapy. JAMA Ophthalmol. 2014;132:1453-60.
- [CrossRef] [PubMed] [Google Scholar]
- Recommendations on screening for chloroquine and hydroxychloroquine retinopathy (2016 revision) Ophthalmology. 2016;123:1386-94.
- [CrossRef] [PubMed] [Google Scholar]
- Hydroxychloroquine and Chloroquine Retinopathy: Recommendations on Monitoring. 2020. https://www.rcophth.ac.uk/wpcontent/uploads/2020/02/HCR-recommendations-onmonitoring-executive-summary.pdf [Last accessed on 2020 Jul 20]
- [Google Scholar]






