Translate this page into:
Management of psoriatic arthritis
*Corresponding author: Dr. Sumi Thomas, Department of Dermatology, Aster Dr. Moopen’s Polyclinic, Al Muteena, Deira, Dubai, United Arab Emirates. sumithomas1@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Thomas S. Management of psoriatic arthritis. J Skin Sex Transm Dis 2019;1:13-8.
Abstract
The treatment of psoriatic arthritis (PsA) makes use of many agents. Most of them are used for the treatment of other forms of inflammatory arthritis or the management of cutaneous manifestations of psoriasis. Although a number of medications are effective in the treatment of both rheumatoid arthritis (RA) and PsA, trials involving some classes of biologic agents indicate that patients with RA and PsA may show different responses to certain drug classes. Treatment of the different elements of PsA includes coordinated interventions to address the major domains of the disease, including peripheral and axial arthritis, enthesitis, dactylitis, and skin and nail involvement.
Keywords
Psoriatic arthritis
Treatment
Biological agents
INTRODUCTION
Psoriatic arthritis (PsA) is inflammatory arthritis seen in association with psoriasis.[1] It is usually seronegative arthritis.
PsA affects men and women equally with an incidence of approximately 6/100,000/year.[1,2] Prevalence of PsA among patients with psoriasis ranges from 4 to 30%.[3,4]
PsA affects peripheral joints, axial joints, or both. Enthesitis, tenosynovitis, and dactylitis may also occur.
There are no laboratory findings that are characteristic of PsA and distinguish it from other forms of inflammatory arthritis.
DIFFERENTIAL DIAGNOSIS
Rheumatoid arthritis (RA)
Patients with PsA in the form of a polyarthritis can present in a fashion that may be indistinguishable from RA. However, the involvement of the distal interphalangeal (DIP) joints, an asymmetric distribution of joint disease, spondyloarthritis, sausage digits, new bone formation on radiographs, cutaneous findings, and the characteristic nail manifestations of PsA all help to distinguish it from RA.[5] Only a small number of patients with PsA will test positive for rheumatoid factor or anti-citrullinated peptide antibodies, while these tests are positive in a majority of patients with RA.
Reactive arthritis
Asymmetric oligoarthritis, enthesitis, sausage digits, and back pain can be seen in patients with either reactive arthritis (formerly called Reiter syndrome) or PsA. However, unlike reactive arthritis, a history of an antecedent infectious illness, with either genitourinary symptoms of urethritis or a dysenteric illness, would not be characteristic of PsA. In addition, human leukocyte antigen-B27 positivity occurs more commonly in patients with reactive arthritis.
Arthritis of inflammatory bowel disease
The peripheral and axial arthritis associated with inflammatory bowel disease may occur in a very similar pattern to that seen in PsA; both diseases may manifest as symmetric or asymmetric oligoarthritis or sacroiliitis. Uveitis may be seen in both and the frequency of psoriasis may be increased in patients with Crohn’s disease. However, inflammatory bowel disease can usually be suspected based on clinical findings and confirmed with endoscopic investigation and biopsy.
Ankylosing spondylitis
A spinal disease that occurs in patients with PsA may be difficult to distinguish from ankylosing spondylitis.[5,6] Peripheral features of PsA such as enthesitis and dactylitis can occur in either condition. However, the presence of psoriasis and some radiographic features may help distinguish the two disorders. PsA is more often associated with the presence of asymmetric sacroiliitis, non-marginal syndesmophytes, asymmetric syndesmophytes, more frequent involvement of the cervical spine, and less frequent involvement of the lumbar spine.
Gout
Both PsA and gout can present as an acute monoarthritis or oligoarthritis, and psoriasis can be associated with hyperuricemia. The diagnosis of gout can be confirmed by finding monosodium urate crystals on examination of the synovial fluid.
Osteoarthritis
Both PsA and osteoarthritis may be characterized by involvement of the DIP joints. PsA as the cause of DIP joint involvement can generally be distinguished from osteoarthritis with Heberden’s nodes by the nodular bony change of osteoarthritis, the more diffuse swelling of the joint in PsA, and the presence of other characteristic findings of PsA.
MANAGEMENT OF PsA
The approach to treatment includes therapy for musculoskeletal disease as well as for disease of the skin and nails and is aimed at controlling inflammation and preventing discomfort, joint damage, and disability [7,8] thereby improving the quality of life.
Pre-treatment screening for comorbidities such as diabetes mellitus, dyslipidemia, hypertension, coronary artery disease, and fatty liver is essential.
Screening for hepatitis should be done in patients before initiating methotrexate (MTX) therapy, and screening for latent tuberculosis (TB) should be done before starting biological agents.
NONPHARMACOLOGIC TREATMENT
This includes exercise, physical therapy, and occupational therapy. Patients should be advised about weight reduction and proper eating habits.
Main medications used to treat PsA include [Flow Chart 1]:
NSAIDs
Disease-modifying anti-rheumatic drugs (DMARD)
Biologic therapies.
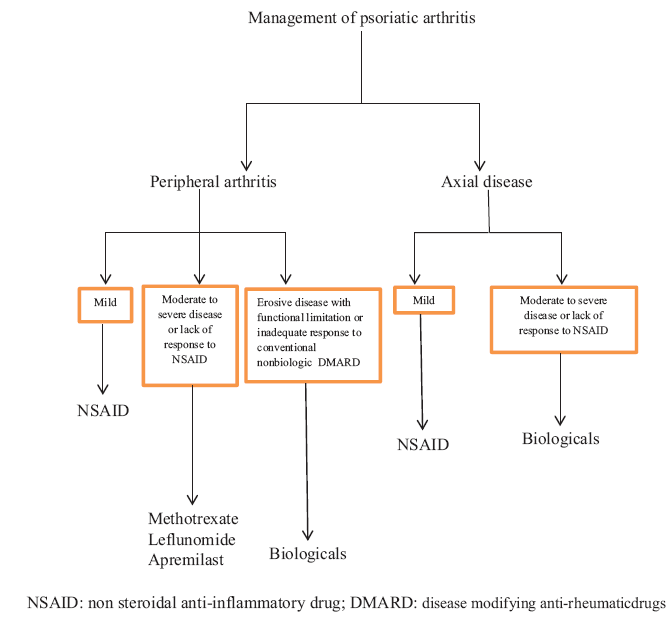
- Management of psoriatic arthritis.
PERIPHERAL ARTHRITIS
Mild arthritis
In patients with mild arthritis, defined as disease involving <4 joints, no radiological evidence of damage, and minimal discomfort or functional impairment, initial treatment can be started with NSAIDs (e.g. naproxen 375–500 mg twice daily, celecoxib 200 mg twice daily, or etoricoxib 60–90 mg daily) rather than starting a DMARD.[7,8] Other NSAIDs that can be used include ibuprofen (up to 2400 mg/day), diclofenac (up to 150 mg/day), or ketoprofen (up to 200 mg/day). Comparative studies did not find any difference in efficacy between different NSAIDs and there was no significant aggravation of skin disease.
MODERATE-TO-SEVERE ARTHRITIS OR RESISTANT TO NSAIDS
In patients whose peripheral arthritis remains active (i.e. persistent joint inflammation) despite the use of NSAIDs, DMARD is the preferred option, rather than a biologic agent.
MTX
MTX (15–25 mg once weekly) can be started in patients with peripheral arthritis not well controlled with NSAIDs. Patients receiving MTX should also be treated with folic acid (1 mg daily) to reduce the risk of adverse effects.
The maximal response to MTX is usually achieved within 3 months of treatment with the drug. Patients who do not respond to MTX 25 mg/week for 6–8 weeks are unlikely to respond to more prolonged therapy. On the other hand, discontinuation of MTX in responders is often associated with severe flares of both skin and joint disease, and hence slow tapering is recommended. The most serious side effects anticipated with MTX are liver toxicity, interstitial lung disease, and bone marrow suppression. The role of liver biopsy in monitoring hepatotoxicity in patients treated with MTX has been controversial. The American Academy of Dermatology and the National Psoriasis Foundation do NOT recommend liver biopsies in all patients who are administered MTX.[9,10]
Leflunomide
LEF (20 mg daily, taken orally) is used in patients who have persistent joint inflammation despite 3 months of treatment with MTX and in patients who are unable to tolerate MTX due to adverse effects, such as liver toxicity. It also improves skin disease but may be less effective for the skin changes than MTX.[11]
Apremilast
In patients with PsA and nonerosive inflammatory arthritis, apremilast (30 mg twice daily following an up-titration at a rate of 10 mg daily over 6 days), a novel, orally administered phosphodiesterase-4 inhibitor, is recommended. The drug modifies multiple pro-inflammatory mediators and cytokines involved in the innate and adaptive immunity.[12]
Apremilast may also be of benefit in patients with enthesitis and dactylitis early in the disease course. It may be particularly appropriate for PsA patients with multiple comorbidities, given the excellent safety profile and documented efficacy in randomized trials for both psoriasis and PsA. It is also useful for patients who wish to avoid DMARD therapy, infusions, or injections. Some patients require up to 4 months of treatment to achieve a maximal response to the drug.
Apremilast should not be used in patients with the erosive disease, as the capacity of apremilast to prevent joint injury has not been established. Routine laboratory monitoring is not required, but baseline serum creatinine is important because the dose should be reduced to 30 mg once daily in patients with creatinine clearance estimated at <30 mL/min.[13]
SEVERE PERIPHERAL ARTHRITIS
In patients whose joint counts do not improve substantially after 3 months of treatment with a conventional nonbiologic DMARD (e.g., MTX) or who already have the erosive disease and functional limitation, a tumor necrosis factor (TNF) inhibitor is recommended.[14] Typically patients require up to 3 months of therapy to achieve a maximal response. Initially, add the TNF inhibitor while continuing the conventional DMARD (e.g. MTX). However, in contrast to RA, MTX generally can be discontinued in PsA patients who respond to TNF-inhibitor therapy, with one important exception, which is infliximab; some experts also prefer that patients receiving adalimumab should also continue MTX.
Other cytokine inhibitors such as anti-interleukin (IL)-12/23 (ustekinumab) and anti-IL-17 (secukinumab and ixekizumab) have also been approved for the treatment of PsA. These agents work extremely well for the skin disease and are especially useful in patients with contraindications to use of anti-TNF agents.[14]
CHOICE OF TNF INHIBITOR
The five original TNF inhibitors and their respective dosing regimens are:
Etanercept
About 50 mg as a subcutaneous injection once weekly, etanercept is a dimeric p75 TNF-alpha receptor Fc fragment fusion protein that binds TNF.
Infliximab
Administered as 5mg/kg by intravenous infusion at 0, 2, and 6 weeks, followed by 5 mg/ kg every 8 weeks thereafter. It may be given either with or without MTX. Infliximab is a human/mouse chimeric anti-TNF-alpha antibody.
Adalimumab
About 40 mg subcutaneously once every 2 weeks, adalimumab is a human monoclonal anti-TNF antibody.
Golimumab
About 50 mg subcutaneously once monthly, golimumab is a human monoclonal anti-TNF antibody.
Certolizumab pegol
Initial dose of 400 mg (administered as two 200 mg injections subcutaneously), repeated 2 and 4 weeks after the initial dose; maintenance is with 200 mg once every other week, or 400 mg every 4 weeks. Certolizumab pegol is a pegylated Fab fragment of a humanized anti-TNF monoclonal antibody.
Screening for latent TB before beginning therapy with anti-TNF agents is necessary, and those with evidence of disease usually require prophylactic anti-TB therapy. TNF inhibitors are usually avoided in patients with first-degree relatives with multiple sclerosis.
Sustained benefit from these drugs has been shown among patients with PsA according to the various studies.[15,16] In addition to improvement in clinical signs and symptoms, these agents also reduce radiographic progression of disease.[17] The efficacy of these drugs for PsA appears comparable, and the choice of the agent is based on a patient preference for route and frequency of administration and the potential cost to the patient.
PERIPHERAL ARTHRITIS RESISTANT TO INITIAL TNF INHIBITOR
Patients who experience an inadequate response to the first TNF inhibitor used can be switched to a second TNF inhibitor rather than trying a different class of biologic agent.
In patients who do not respond adequately to two different TNF inhibitors, its recommended to use an IL-17 inhibitor (i.e., secukinumab or ixekizumab) or the IL-12/23 inhibitor ustekinumab.
Anti-IL-17 therapies
Several drugs that inhibit IL-17 are available for use in PsA and/ or psoriasis
Secukinumab
A human anti-IL-17A monoclonal antibody is available for the treatment of PsA and psoriasis. It was shown to be effective for the treatment of PsA in a series of phase 3 randomized trials.[18] Secukinumab is administered by subcutaneous injection, usually with a loading dose of 150 mg at weeks 0, 1, 2, 3, and 4, followed by 150 mg every 4 weeks; it may be increased to 300 mg every 4 weeks in patients who have failed TNF inhibitors or who continue to have active arthritis despite 150 mg every 4 weeks. In patients with severe psoriasis, the usual dose is 300 mg every 4 weeks.
Ixekizumab
Ixekizumab is also an IL-17A monoclonal antibody, which demonstrated efficacy in both psoriasis and PsA.[19] Ixekizumab 160 mg initially, then 80 mg every 2 or 4 weeks by subcutaneous injection is the dosage used.
Brodalumab
An anti-IL-17 receptor antibody that is approved by the US Food and Drug Administration for use in psoriasis and has shown efficacy in trials for PsA.[20]
Ustekinumab
A human monoclonal antibody directed against the shared p40 subunit of interleukin 12 and interleukin 23, which interferes with receptor binding to immune cells. It is commercially available for the treatment of both psoriasis and PsA and is administered by subcutaneous injection (45 mg, given initially and 4 weeks later, then every 12 weeks). The efficacy and safety of ustekinumab for PsA have been demonstrated in several randomized trials, which included patients who had active PsA despite having received either NSAIDs, a conventional nonbiologic DMARD, or a TNF inhibitor.[21]
Abatacept
In patients who have not responded adequately to TNF inhibitor therapy and other biologics such as secukinumab and ustekinumab, another treatment option is abatacept (CTLA4-Ig), a selective T-cell costimulation modulator, used for the treatment of RA. It has been used in the treatment of PsA since 2017;[22] dosage is 125 mg by subcutaneous injection once weekly.
JANUS KINASE (JAK) INHIBITORS
Tofacitinib
An oral inhibitor of JAK has demonstrated efficacy in the treatment of PsA in several randomized trials, including both patients with an inadequate response to a conventional synthetic DMARD[23] and patients with an inadequate response to TNF inhibitor therapy.[24] Tofacitinib inhibits cytokine pathways important in PsA and psoriasis through its effects on JAK3 and JAK1. The dosage is 5 mg or 10 mg twice daily.
Other JAK inhibitors, including the selective JAK1 inhibitor and filgotinib, are under investigation. Dosage is 200 mg orally once daily.[25]
AXIAL DISEASE
The choice of therapy for axial disease (i.e. involving the sacroiliac joints and spine) is based on the severity of disease and the patient’s response to treatment. Patients with mild symptoms may be effectively treated by use of NSAIDs, while patients with moderate-to-severe arthritis or who are resistant to NSAIDs alone are usually treated with a TNF inhibitor rather than a traditional non-biologic DMARD, as the latter is found to be ineffective for spondylitis.[14] Biologic agents such as secukinumab or ustekinumab are also effective in the treatment of axial disease.
Alternative conventional DMARDs such as sulfasalazine,[26] azathioprine, or cyclosporine are reserved for patients with PsA who do not respond or have toxic reactions to the usual medications. Systemic corticosteroids are not preferred owing to side effects associated with long-term use and also for fear of the development of erythroderma or pustular psoriasis on withdrawal; however, intra-articular glucocorticoids are found useful as adjunctive therapy.[14] Although hydroxychloroquine has shown benefit in the treatment of PsA, its not a preferred drug, due to the risk of aggravation of skin disease.[27] Anakinra, an IL-1 receptor antagonist, has shown modest benefit in PsA.[28]
Risk factors for progressive joint damage
The factors predicting a poor prognosis with respect to progression of peripheral joint injury are:[29]
Increased numbers of actively inflamed joints
Elevated erythrocyte sedimentation rate or C-reactive protein
Failure of previous medication trials
The presence of joint damage (clinically or radiographically)
Loss of function (by Health Assessment Questionnaire)
Diminished quality of life.
Remission
Complete relief of joint tenderness and swelling may occur in a substantial minority of treated patients. In one study involving 391 patients, 69 (18%) achieved remission, and nearly one-half remained free of active joint disease without continued use of medication.[30]
Clinical monitoring of disease should include joint counts that assess both the upper and lower extremities and the number of areas involved by enthesitis and by dactylitis. In clinical practice, plain film radiography of clinically involved peripheral joints, the sacroiliac joints, and the spine is used to assess the extent and progression of disease at these sites.
CONCLUSION
PsA was once considered a mild disease for which clinicians were reluctant to use DMARDs; however, it has since become clear that the disease is more severe than previously described[31] and a significant proportion of patients may develop destructive and potentially disabling disease. Hence, treatment of PsA should be started early in the disease and should be coordinated between a rheumatologist and a dermatologist.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Diagnosis and management of psoriatic arthritis. Drugs. 2002;62:2447-57.
- [CrossRef] [PubMed] [Google Scholar]
- Current concepts in psoriatic arthritis. Curr Opin Rheumatol. 2002;14:361-6.
- [CrossRef] [PubMed] [Google Scholar]
- Epidemiology of psoriatic arthritis in the population of the United States. J Am Acad Dermatol. 2005;53:573.
- [CrossRef] [PubMed] [Google Scholar]
- Comparative performance of psoriatic arthritis screening tools in patients with psoriasis in European/North American dermatology clinics. J Am Acad Dermatol. 2014;71:649-55.
- [CrossRef] [PubMed] [Google Scholar]
- Psoriatic arthritis: Current concepts on pathogenesis-oriented therapeutic options. Arthritis Rheum. 2007;56:1051-66.
- [CrossRef] [PubMed] [Google Scholar]
- Classification and diagnostic criteria for psoriatic arthritis. Ann Rheum Dis. 2005;64(Suppl 2):ii3-8.
- [CrossRef] [Google Scholar]
- Psoriatic arthritis: Recognition and management. BioDrugs. 1998;9:271-8.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment of psoriatic arthritis. Baillieres Clin Rheumatol. 1994;8:483-98.
- [CrossRef] [Google Scholar]
- Methotrexate and psoriasis: 2009 national psoriasis foundation consensus conference. J Am Acad Dermatol. 2009;60:824-37.
- [CrossRef] [PubMed] [Google Scholar]
- Guidelines of care for the management of psoriasis and psoriatic arthritis: Section 4. Guidelines of care for the management and treatment of psoriasis with traditional systemic agents. J Am Acad Dermatol. 2009;61:451-85.
- [CrossRef] [PubMed] [Google Scholar]
- The effectiveness of leflunomide in psoriatic arthritis. Clin Exp Rheumatol. 2014;32:728-31.
- [Google Scholar]
- Apremilast, a cAMP phosphodiesterase-4 inhibitor, demonstrates anti-inflammatory activity in vitro and in a model of psoriasis. Br J Pharmacol. 2010;159:842-55.
- [CrossRef] [PubMed] [Google Scholar]
- Impact of renal impairment on the pharmacokinetics of apremilast and metabolite M12. Clin Pharmacol Drug Dev. 2016;5:469-79.
- [CrossRef] [PubMed] [Google Scholar]
- European league against rheumatism (EULAR) recommendations for the management of psoriatic arthritis with pharmacological therapies: 2015 update. Ann Rheum Dis. 2016;75:499-510.
- [CrossRef] [PubMed] [Google Scholar]
- The comparative one-year performance of anti-tumor necrosis factor alpha drugs in patients with rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis: Results from a longitudinal, observational, multicenter study. Arthritis Rheum. 2008;59:234-40.
- [CrossRef] [PubMed] [Google Scholar]
- Sustained clinical response in psoriatic arthritis patients treated with anti-TNF agents: A 5-year open-label observational cohort study. Semin Arthritis Rheum. 2011;40:398-406.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of tumour necrosis factor blockers on radiographic progression of psoriatic arthritis: A systematic review and meta-analysis of randomised controlled trials. Ann Rheum Dis. 2014;73:414-9.
- [CrossRef] [PubMed] [Google Scholar]
- Secukinumab inhibition of interleukin-17A in patients with psoriatic arthritis. N Engl J Med. 2015;373:1329-39.
- [CrossRef] [PubMed] [Google Scholar]
- Ixekizumab for the treatment of patients with active psoriatic arthritis and an inadequate response to tumour necrosis factor inhibitors: Results from the 24-week randomised, double-blind, placebo-controlled period of the SPIRIT-P2 phase 3 trial. Lancet. 2017;389:2317-27.
- [CrossRef] [Google Scholar]
- Brodalumab, an anti-IL17RA monoclonal antibody, in psoriatic arthritis. N Engl J Med. 2014;370:2295-306.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy and safety of ustekinumab in patients with active psoriatic arthritis: 1 year results of the phase 3, multicentre, double-blind, placebo-controlled PSUMMIT 1 trial. Lancet. 2013;382:780-9.
- [CrossRef] [Google Scholar]
- Efficacy and safety of abatacept, a T-cell modulator, in a randomised, double-blind, placebo-controlled, phase III study in psoriatic arthritis. Ann Rheum Dis. 2017;76:1550-8.
- [CrossRef] [PubMed] [Google Scholar]
- Tofacitinib or adalimumab versus placebo for psoriatic arthritis. N Engl J Med. 2017;377:1537-50.
- [CrossRef] [PubMed] [Google Scholar]
- Tofacitinib for psoriatic arthritis in patients with an inadequate response to TNF inhibitors. N Engl J Med. 2017;377:1525-36.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy and safety of filgotinib, a selective Janus Kinase 1 inhibitor, in patients with active psoriatic arthritis (EQUATOR): Results from a randomised, placebo-controlled, phase 2 trial. Lancet. 2018;392:2367-77.
- [CrossRef] [Google Scholar]
- Sulfasalazine in the treatment of spondylarthropathy. A randomized, multicenter, double-blind, placebo-controlled study. Arthritis Rheum. 1995;38:618-27.
- [CrossRef] [PubMed] [Google Scholar]
- Hydroxychloroquine in psoriatic arthropathy: Exacerbations of psoriatic skin lesions. J Rheumatol. 1982;9:462-4.
- [Google Scholar]
- Anakinra (Kineret) in psoriasis and psoriatic arthritis: A single-center, open-label, pilot study. Arthritis Res Ther. 2005;7(Suppl 1):P68.
- [CrossRef] [Google Scholar]
- Treatment recommendations for psoriatic arthritis. Ann Rheum Dis. 2009;68:1387-94.
- [CrossRef] [PubMed] [Google Scholar]






