Translate this page into:
Sensitivity, specificity, and positive predictive value of Tzanck smear in diagnosing intraepidermal immunobullous diseases in patients presenting with immunobullous diseases
*Corresponding author: Dr. Nirmal Chandrasekhar, Sreehari, Old Beypore Road, Arakkinar P.O, Kozhikode - 673 028, Kerala, India. nirmalchandrasekhar@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Chandrasekhar N, Abdul Latheef EN. Sensitivity, specificity, and positive predictive value of Tzanck smear in diagnosing intraepidermal immunobullous diseases in patients presenting with immunobullous diseases. J Skin Sex Transm Dis 2019;1:26-9.
Sir,
Immunobullous disorders are characterized by blistering of skin and mucosae induced by antibodies against desmoglein 1 and 3 in pemphigus group, hemidesmosomes, and components of basement membrane zone in pemphigoid group and transglutaminases in dermatitis herpetiformis. Based on the level of cleavage which, in turn, is determined by the site of target antigen, immunobullous disorders are broadly classified into intraepidermal and subepidermal types.
Intraepidermal blistering is seen in pemphigus group of diseases. Acantholytic blisters within the epidermis are a characteristic feature of pemphigus.
Accurate differentiation between intraepidermal and subepidermal immunobullous diseases requires histopathologic evaluation and to arrive at the exact diagnosis direct immunofluorescence (DIF) study is often needed. Diagnosis based on histopathology and DIF requires an inevitable delay of couple of days. In this scenario, Tzanck smear (a cytodiagnostic technique) that evaluates acantholysis, which is the pathogenic mechanism in intraepidermal immunobullous diseases offers an easy, inexpensive, and rapid screening test that could differentiate intraepidermal from non-intraepidermal immunobullous diseases.
In this cross-sectional study, we aimed to determine the sensitivity, specificity, and positive predictive value of Tzanck smear in diagnosing intraepidermal immunobullous diseases in patients >5 years of age who attended the dermatology department of a tertiary referral center from March 1, 2011 to February 29, 2012 with immunobullous diseases. Clearance from institutional ethics committee and written informed consent from individual study subject were obtained.
Patients who were not willing to participate in the study were excluded from the study.
Using a pre-set pro forma, patient data including age, gender, duration and evolution of disease, history of previous medication, and clinical profile were collected.
At the time of presentation, a clinical diagnosis into pemphigus or pemphigoid group of disease was made based on the morphology, distribution of skin lesions, presence or absence of mucosal lesions, and Nikolsky and bulla-spread or Asboe-Hansen sign.
Tzanck smear analysis was performed in all patients using standard protocol. When acantholytic cells were seen, the patient was considered to have intraepidermal immunobullous disease.
Skin biopsy from lesional skin (from intact vesicle or bulla) was evaluated (by pathologist and histopathology diagnosis was recorded) in each patient. Patients who showed suprabasal clefting with row of tombstone appearance/subcorneal clefting and presence of acantholytic cells in blister cavity were classified as intraepidermal immunobullous diseases and those who did not reveal the same were categorized as subepidermal immunobullous diseases.
The histopathologic diagnosis was compared with the Tzanck smear study, after entering the data into Microsoft Excel. Sensitivity, specificity, and positive predictive value of Tzanck study in diagnosing intraepidermal immunobullous diseases in patients presenting with immunobullous disease were determined.
The study population comprised 50 patients with a male-to-female ratio of 0.85:1 {23 males (46%) and 27 females (54%)}.
Age of the study subjects ranged from 18 to 90 years. 62% of the affected was in the 30–60 age groups. 12% of patients were <30 years and the remaining 26% were >60 years; youngest and eldest patients were female.
The sites of the distribution of lesions in the study group are depicted in Table 1.
| Immunobullous diseases | Sites of lesions | ||||||
|---|---|---|---|---|---|---|---|
| Scalp (%) | Face and neck (%) | Trunk (%) | Extremities (%) | Oral lesions (%) | Genital lesions (%) | Nail involvement (%) | |
| Pemphigus group (30) | 29 (96.7) | 30 (100) | 30 (100) | 26 (86.7) | 24 (80) | 11 (36.7) | 17 (56.7) |
| Pemphigoid group (20) | 0 (0) | 10 (50) | 18 (95) | 19 (95) | 6 (30) | 5 (25) | 10 (50) |
| Total | 29 (58) | 40 (80) | 48 (96) | 45 (90) | 30 (60) | 16 (32) | 27 (54) |
Thirty patients clinically manifested as pemphigus group of disease [Figures 1a-3a] and 20 patients pemphigoid group of disease [Figures 4a and b].

- (a) Flaccid bullae and erosions, (b) Skin biopsy from flaccid bulla showing suprabasal cleavage (H and E, ×100), (c) Acantholytic cell in the same histology specimen (H and E, ×400).

- (a) Crusted erosions in a patient presenting with immunobullous disease, (b) Skin biopsy showing subcorneal cleavage (H and E, ×100).

- (a) Vegetative plaques in a patient presenting with immunobullous disease, (b) Skin biopsy showing irregular acanthosis and intraepidermal bulla (H and E, ×100), (c) Eosinophils and acantholytic cells within the bulla cavity (H and E, ×400).

- (a) Tense vesicles and blister in a patient presenting with immunobullous disease, (b) String of pearl appearance in another patient presenting with immunobullous disease, (c) Skin biopsy revealing subepidermal bulla (H and E, ×40).
HISTOLOGY
Thirty-one patients were categorized as having intraepidermal immunobullous diseases by histopathologic analysis [Figures 1b-3b]. 17 had subepidermal blistering [Figure 4c], and the remaining two showed neutrophilic microabscess in the dermal papillae on histopathology analysis.
Among the 31 intraepidermal immunobullous disease patients, 83.9% had suprabasal separation [Figure 1b] and 16.1% had subcorneal clefting [Figure 2b].
Acantholytic cells [Figures 1c and 3c] were noted in the histopathology of 28 intraepidermal immunobullous cases.
Clinicohistological concordance was observed in 49/50 (98%) patients since one patient with clinical features suggestive of pemphigoid group of disease manifested intraepidermal bullae.
TZANCK SMEAR
Tzanck smear showed acantholytic cells [Figure 5a] in 24/50 (48%) cases and all of them belonged to the clinical and histological category of intraepidermal immunobullous diseases. The clinically categorized pemphigoid group of patient who manifested intraepidermal bulla did not show any acantholytic cells. None of the subepidermal immunobullous group of diseases had acantholytic cells.
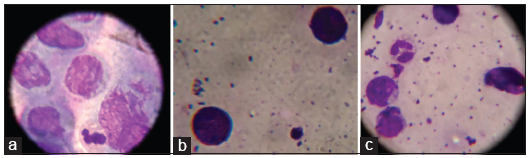
- (a) Acantholytic cells in Tzanck smear (Leishman ×1000), (b) Eosinophils in Tzanck smear (Leishman ×1000), (c) Lymphocytes and neutrophils in Tzanck smear (Leishman ×1000).
Sensitivity of Tzanck smear in identifying intraepidermal immunobullous group of diseases (24/31) was 77.4%. Both the specificity and positive predictive value of Tzanck study in diagnosing the same (no false positivity was documented in the study group) were 100%. Lymphocytes, neutrophils, and eosinophils were also observed in Tzanck smear of different patients [Figures 5b and c].
Tzanck smear, a method of cytodiagnosis was first described by George Papanicolaou.[1] However, it was Arnault Tzanck, who first used it in the diagnosis of dermatology conditions.[2] The sensitivity of Tzanck smear in diagnosing pemphigus was expected since it assesses the effect of acantholysis, the pathogenic mechanism in the disease. The term acantholysis was coined by Auspitz.[3]
Acantholytic cell is a large, rounded keratinocyte with hypertrophic nucleus, hazy or absent nucleoli, and abundant basophilic cytoplasm. The cell shows a perinuclear halo due to condensation of cytoplasm at the periphery.[1]
Acantholytic cells in Tzanck smear are not specific for intraepidermal immunobullous diseases. It is seen in blisters due to viral infections, burns, Stevens-Johnson syndrome-toxic epidermal necrolysis, etc.
We limited the study to children >5 years since some of the intraepidermal and subepidermal bullous diseases that appear in early life belong to the epidermolysis bullosa group which are mechanobullous rather than immunobullous group of diseases. They also include intraepidermal and subepidermal group of diseases, but both lack acantholytic cells since the mechanism of bulla formation is different.
The age and sex distribution, and the distribution of lesions observed in the study group were comparable to the previous studies.[4-8]
We noted 98% of clinicohistopathological correlation in immunobullous group of patients. This was higher than most other studies that recorded a concordance rate of 85–87%.[5-7] 100% concordance between clinical and histopathology findings in pemphigus group of diseases as noted by us was consistent with another Indian study.[6]
Intraepidermal separation observed in one patient whose clinical features were suggestive of pemphigoid group of disease was as reported in literature and is attributed to reepithelialization of floor of blister which may be seen when biopsy is taken from an old lesion.[9]
Our observation of 77.4% intraepidermal immunobullous diseases manifesting acantholytic cells was consistent with one Indian study, but certain studies recorded 50–100% positivity for acantholytic cells in pemphigus.[1,10,11] 100% specificity for the presence of acantholytic cells in Tzanck smear to rule out subepidermal blistering diseases as observed by us was comparable to one previous study.[10]
Small sample size and lack of DIF analysis were the major limitations of this study.
Tzanck smear analysis, which is a simple technique that gives immediate results, is a useful test to rule out subepidermal immunobullous diseases among patients presenting with immunobullous diseases.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Diagnostic utility and pitfalls of tzanck smear cytology in diagnosis of various cutaneous lesions. J Cytol. 2017;34:179-82.
- [CrossRef] [PubMed] [Google Scholar]
- The man behind the eponym. Arnault Tzanck, his work and times. Am J Dermato Pathol. 1985;7:121-3.
- [CrossRef] [Google Scholar]
- Acantholysis revisited: Back to basics. Indian J Dermatol Venereol Leprol. 2013;79:120-6.
- [CrossRef] [PubMed] [Google Scholar]
- Immunobullous disorders: Clinical histopathological and immunofluorescence study of thirty-six cases. Muller J Med Sci Res. 2014;5:134-8.
- [CrossRef] [Google Scholar]
- A clinico-pathological study of 70 cases of pemphigus. Indian J Dermatol Venereol Leprol. 1999;65:168-71.
- [Google Scholar]
- A retrospective study of the clinical, histopathological, and direct immunofluorescence spectrum of immunobullous disorders. Egypt J Dermatol Venereol. 2017;37:62-8.
- [CrossRef] [Google Scholar]
- Clinical, demographic and immunopathological spectrum of subepidermal autoimmune bullous diseases at a tertiary center: A 1-year audit. Indian J Dermatol Venereol Leprol. 2016;82:358.
- [CrossRef] [PubMed] [Google Scholar]
- Spongiotic intra epidermal blister: A pitfall in the histopathologic diagnosis of bullous pemphigoid. Indian J Dermatol. 2013;58:410.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnostic value of Tzanck smear in various erosive, vesicular, and bullous skin lesions. Indian Dermatol Online J. 2015;6:381-6.
- [CrossRef] [PubMed] [Google Scholar]
- Pemphigus vulgaris of skin: Cytological findings and pitfalls. Acta Cytol. 2012;56:310-4.
- [CrossRef] [PubMed] [Google Scholar]





