Translate this page into:
Type 2 lepra reaction presenting as nasal septal perforation: A case report
*Corresponding author: P. Jishna, Assistant Professor, Department of Dermatology and Venereology, Goverment Medical College, Kozhikode, Kerala, India. jishnapvijayan@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Nadeem A, Jishna P, Sasidharanpillai S, Bindu V, Vidya A, Rahima S. Type 2 lepra reaction presenting as nasal septal perforation: A case report. J Skin Sex Transm Dis 2020;2(2):137-9.
Sir,
Lepra reactions, the acute exacerbations occurring during the otherwise chronic course of leprosy, are an important cause for deformities. Reactions can occur before, during, or after completion of treatment of leprosy. Reactions occurring after completion of multidrug therapy (MDT) are designated as late lepra reactions where it often poses difficulty in differentiating from leprosy relapse. We report late type 2 lepra reaction (T2R) presenting as nasal septal perforation in a patient who successfully completed MDT for lepromatous leprosy (LL).
A 21-year-old male patient attended our institution with right-sided nasal block, pain, and nose bleed of 2-week duration not responding to antihistamines and antibiotics. He was also suffering from high-grade fever, myalgia, and arthralgia. His medical history revealed 1-year treatment with the World Health Organization multibacillary MDT[1] for LL, 3 years back and recurrent T2R with erythema nodosum leprosum (ENL) lesions since 2 months of MDT. The reaction was managed with prednisolone, but reducing the dose of prednisolone below 15 mg always brought out recurrences that continued even after completion of MDT. At the completion of treatment, bacteriological index (BI) of earlobe and slit-skin smears were 3+ and 2+, respectively, whereas MI was zero in both smears. Post-MDT T2R was managed with 15 mg prednisolone on alternate days and 50 mg of clofazimine as the patient declined the option of thalidomide. He did not give any history of bronchial asthma or substance abuse and denied history of treatment with intranasal medications or nasal trauma or nasal surgery.
His complete hemogram showed neutrophilic leukocytosis, thrombocytosis, and elevated erythrocyte sedimentation rate without any evidence of eosinophilia which was confirmed by peripheral smear analysis. Random blood sugar estimation and liver and renal function tests were within normal limits. Antinuclear antibody profile, antineutrophil antibodies, and serology for syphilis and human immunodeficiency virus infection were negative.
Earlobe smear revealed BI 1+ and MI zero. Multiple slit-skin smears performed detected MI zero and BI 1+. Anterior rhinoscopy revealed a mass lesion in the right nasal cavity. Contrast-enhanced computerized tomogram showed a moderately enhancing space-occupying lesion in the right nasal cavity extending to the left side, perforating the nasal septum [Figure 1]. A tissue biopsy of the lesion stained with hematoxylin and eosin showed macrophage granuloma [Figure 2a] with plenty of lymphocytes and a few neutrophils [Figure 2b]. Wade-Fite staining failed to demonstrate any acid-fast bacilli (AFB). By this time, he started developing ENL lesions [Figure 3] on the trunk and proximal extremities.
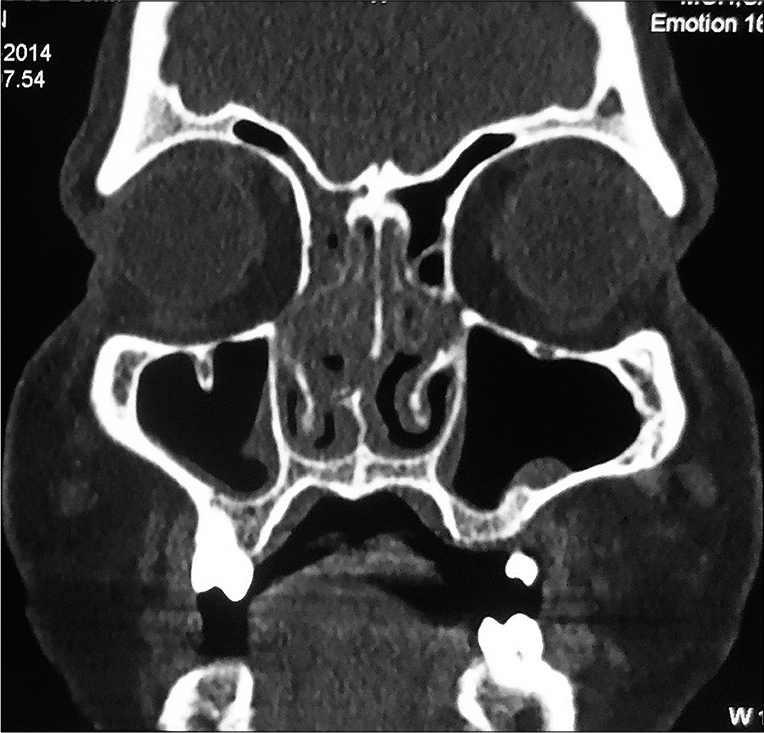
- Contrast-enhanced computerized tomogram of head and neck showing moderately enhancing space-occupying lesion in the right nasal cavity extending to the left side, perforating the nasal septum.
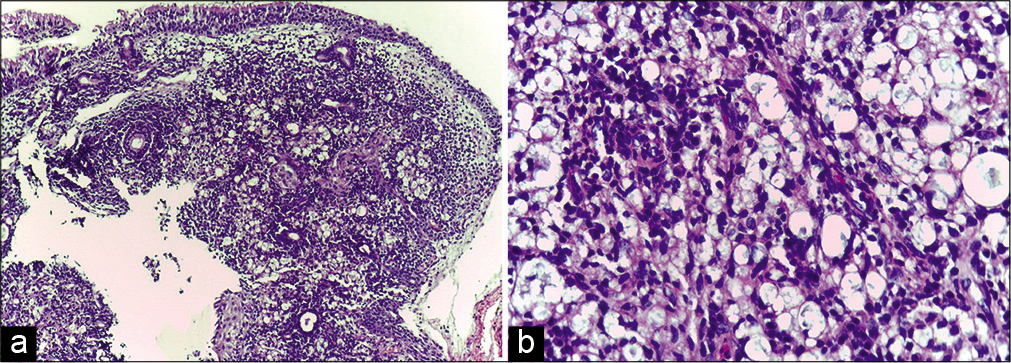
- (a) Biopsy of the nasal cavity lesion showing macrophage granuloma with inflammatory infiltrate (H and E, ×100); (b) High-power view of the same showing inflammatory infiltrate composed of lymphocytes and a few neutrophils (H and E, ×400).
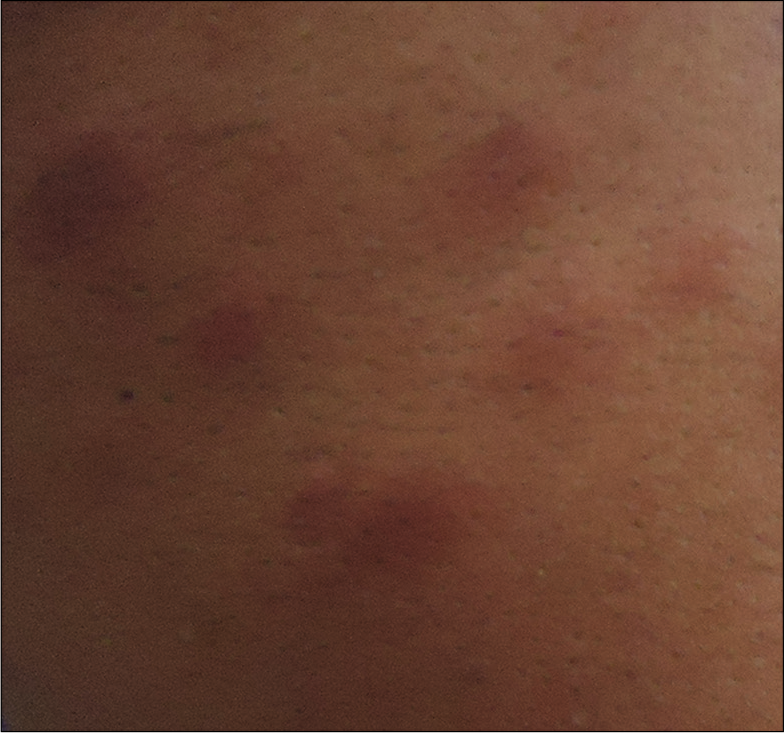
- Erythema nodosum leprosum lesions on the trunk of the same patient.
With the differential diagnoses of LL relapse with T2R and late T2R leading to nasal septal perforation, we opted for a therapeutic trial with 1 mg/kg body weight prednisolone and clofazimine (100 mg 3 time daily). The patient responded with resolution of symptoms within a week confirming the possibility of late T2R. Now after 3 months, he remains asymptomatic; anterior rhinoscopy revealed near-complete resolution of mass lesion. He is currently receiving 10 mg prednisolone and 100 mg clofazimine which we plan to taper off slowly.
Nasal septal perforations mostly remain asymptomatic except for large lesions that manifest as pain, bleeding, runny nose, nasal obstruction, and headache. The various causes for septal perforation are iatrogenic laceration of mucoperichondrium during septoplasty, post-surgical hematoma formation compromising the nutrition of quadrangular septal cartilage, granulomatous diseases such as leprosy and Wegener’s disease and syphilis, trauma, use of narcotics such as cocaine, and drugs such as nasal corticosteroids and nasal vasoconstrictors.[2] Thorough evaluation ruled out most of these causes in our patient.
Infiltration of nasal mucosae by Mycobacterium leprae is well documented and many consider it as the entry and elimination point of the bacillae. Martins et al. observed persistence or worsening of nasal mucosal lesions (as noted in our case) even after completion of treatment. They hypothesized that reduced blood circulation associated with vasculitis (as in T2R) can impair the efficacy of multidrug treatment, thus enabling the bacillus to retain viability with the progression of nasal lesions.[3] This seems unlikely in our patient since the nasal biopsy failed to detect any AFB. Moreover, systemic steroids without MDT would not have attained a lasting resolution had there been viable bacilli. Failure to visualize fragmented AFB in nasal biopsy specimen in our case could be explained by the upgrading of cell-mediated immunity documented in T2R (especially in the treated) leading to rapid clearance of bacilli.[4] The abundance of lymphocytes noted in the histology analysis supports this explanation.
Gradual appearance of new skin lesions or new nerve involvement or presence of live bacilli or a 2 log increase in BI (from previous status) in smear studies are the features favoring a diagnosis of relapse. Acute constitutional symptoms with the appearance of crops of new lesions and sudden involvement of multiple nerves are indicative of T2R. Distinguishing leprosy relapse with reaction from late reaction is often a diagnostic challenge. Therapeutic trial with systemic steroids has been found to be diagnostic when in doubt.[5] Rapid and complete resolution of lesions in response to steroids noted in our patient was more indicative of late T2R rather than T2R with relapse.
ENL involving the nasal septum can manifest as nasal block, epistaxis, and septal perforation.[6] T2R is well known for recurrences even after completion of the treatment as complete clearance of mycobacterial antigen may take 3–4 years as was evident in our case.[7] The common histopathological features noted in ENL are intense neutrophil infiltration with or without vasculitis superimposed on a lepromatous granuloma.[4,5] Lymphocytes may replace neutrophils in resolving lesions. Coexistence of neutrophils with lymphocytes in our patient could be attributed to the delay between the onset of symptoms and the time biopsy.[4]
With the declared elimination of leprosy, the focus is now shifted to diagnosis and management of complications and sequela of the disease in the treated. Late reactions assume significance for the difficulty in distinguishing them from relapse and for being an important cause for deformity in the treated.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
Dr. Saleem Rahima and Dr. Sarita Sasidharanpillai are on the Editorial Board of the Journal.
References
- Training Manual for Medical Officers: NLEP. Ch 7. In: Classification and Management of Leprosy. New Delhi: Directorate of Health Services, Ministry of Health and Family Welfare; Available from: http://wwww.nlep.nic.in/training.html [Last accessed on 2009 Feb 18]
- [Google Scholar]
- Perforation septum: Etiology and diagnosis. Int Arch Otorhinolaryngol. 2010;14:467-71.
- [CrossRef] [Google Scholar]
- Aten year historic study of paranasal cavity endoscopy in patients with leprosy. Rev Bras Otolaringol. 2005;71:609-16.
- [CrossRef] [Google Scholar]
- Leprosy reactions. In: Kar HK, Kumar B, eds. IAL Textbook of Leprosy (1st ed). New Delhi: Jaypee Brothers Medical Publishers (P) Limited; 2010. p. :269-89.
- [CrossRef] [Google Scholar]
- A study on histological features of Lepra reactions in patients attending the dermatology department of the government medical college, Calicut, Kerala, India. Lepr Rev. 2013;84:51-64.
- [Google Scholar]
- Localized and persistent erythema nodosum leprosum a rare variant? Dermatol Online J. 2008;14:16.
- [Google Scholar]





