Translate this page into:
Herpes zoster following ChAdOx1 nCoV-19 corona virus vaccine (recombinant): A case report
*Corresponding author: Daniel Henry, Department of Dermatology, Usha Memorial Skin and Eye Hospital, Bilaspur, Chhattisgarh, India. danielhenry21@yahoo.com
-
Received: ,
Accepted: ,
How to cite this article: Henry D, Henry D. Herpes zoster following ChAdOx1 nCoV-19 corona virus vaccine (recombinant): A case report. J Skin Sex Transm Dis 2022;4:136-8.
Dear Editor,
The life-threatening complications and the unpredictable disease course of Coronavirus disease 2019 (COVID-19) had prompted the search for vaccines effective against the infection. Severe acute respiratory syndrome coronavirus-2 (SARS CoV-2), the causative agent of COVID-19 is a ribonucleic acid (RNA) virus that shows a high mutation rate.[1] This has added to the difficulty in bringing out an effective vaccine. The currently approved vaccines against COVID-19 are classified into mRNA based vaccines, vaccines based on viral vectors, vaccines based on inactivated SARS CoV-2 viruses, and deoxyribonucleic acid based vaccines.[1]
As the vaccination program has gained momentum, occasional reports of adverse reactions following the same are being reported. The reported adverse cutaneous reactions include delayed large local reactions, local injection site reactions, urticarial eruptions, morbilliform eruptions, pernio/chilblains, cosmetic filler reactions, flares of herpes zoster and herpes simplex, pityriasis rosea-like reactions, erythema multiforme, Stevens-Johnson syndrome, lymphomatoid drug eruption resembling pityriasis lichenoides et varioliformis acuta, genital fixed drug eruption, annular lichen planus, late-onset atopic dermatitis, cutaneous vasculitis, new-onset bullous pemphigoid, and erythema nodosum.[2-5]
India began the vaccination program against COVID-19 on January 16, 2021.[6] The two vaccines offered were CovishieldTM [ChAdOx1 nCoV-19 corona virus vaccine (recombinant) manufactured by Serum Institute of India Pvt Ltd] and Covaxin® (inactivated coronavirus vaccine manufactured by Bharat Biotech Limited).[2] A few reports have started emerging regarding the possible adverse events that followed these vaccines.[5,7,8]
Here we report a patient who developed first episode of herpes zoster following CovishieldTM vaccine.
A 53-year-old woman with no known comorbidities, came to our clinic with pain and multiple fluid-filled lesions on the left upper arm. Patient denied any history of trauma or infections or topical applications prior to the onset of the lesions. She denied recent history of any stressful incidents in life. The lesions developed 5 days after receiving the second dose of CovishieldTM vaccine on her left arm. She neither manifested any constitutional symptoms, nor did she give any previous history of similar illness. She gave no history COVID-19. She had not received herpes zoster vaccine in the past.
Dermatological examination revealed multiple grouped, vesicles and bullae on an erythematous background on the left upper arm [Figure 1]. Tzanck smear showed multinucleated giant cells.
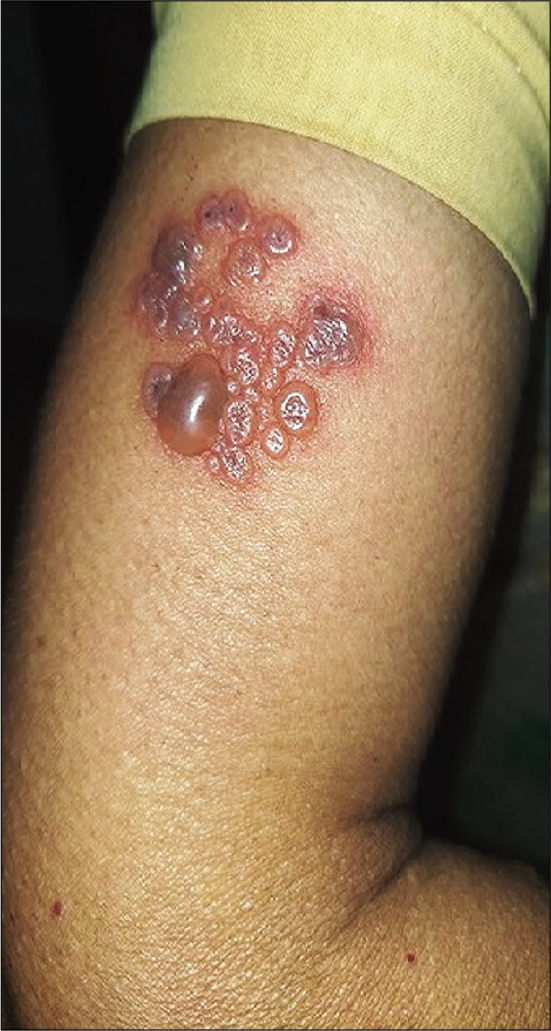
- Multiple grouped vesicular lesions of herpes zoster following COVID-19 vaccination.
The patient was diagnosed as a case of herpes zoster affecting C5 dermatome. We treated her with valacyclovir 1 gm 3 times a day and pregabalin 75 mg at night for 7 days.
Reactivation of herpes zoster and herpes simplex are well reported following mRNA vaccines and Oxford Astrazeneca COVID-19 vaccine.[2] There are a few reports from India on herpes zoster following CovishieldTM and Covaxin®.[8,9] Mohta et al. reported three patients developing reactivation of herpes zoster after CovishieldTM vaccine; all the three patients were receiving cyclosporine for chronic spontaneous urticaria.[9] The patient reported by Arora et al., who manifested herpes zoster after Covaxin, was a 60-year-old man with type 2 diabetes mellitus.[8]
The time interval between COVID-19 vaccination and the appearance of herpes zoster as reported in literature ranged 3–20 days which was similar to the finding in our patient.[5,7-11] Arora et al. and van Dam et al. have reported that patients who had developed herpes zoster after the first dose of Covaxin® and Pfizer-BioNTech COVID-19 mRNA vaccine respectively, subsequently tolerated the second dose without any adverse events or reactivation of herpes zoster.[8,10] Our patient developed herpes zoster after the second dose of CovishieldTM vaccine.
Herpes zoster results from reactivation of varicella-zoster virus. A meta-analysis identified immunosuppression associated with human immunodeficiency virus infection and malignancy, older age group, family history of herpes zoster, and physical trauma as risk factors for herpes zoster.[12] Immunosenescence (immunosuppression that occurs with age) is cited as the reason for increased risk for herpes zoster with advancing age. Herpes zoster vaccine is recommended to all those aged 50 years and above.[13]
COVID-19 itself is documented as a risk factor for herpes zoster.[11] It is suggested that COVID-19-associated lymphocytopenia, especially involving CD3+CD8+ lymphocytes and functional impairment of CD4+ T cells can lead to reactivation of varicella-zoster virus.[11] In the current setting, herpes zoster has been proposed as a probable sign of undiagnosed COVID-19 in younger age groups.[11]
Information on reverse transcription-polymerase chain reaction for COVID-19 was lacking in our patient at the time of manifestation of herpes zoster. Hence we can not rule out the possibility of asymptomatic COVID-19 in our case. Moreover, though not common, herpes zoster can affect immunocompetent individuals as well. The estimated incidence rate of herpes zoster among immunocompetent population aged above 50 years and unvaccinated against the virus is 9.92 per 1000 person years.[14] Hence, we are unable to draw a definite conclusion on the role of CovishieldTM vaccine on the eruption of herpes zoster in our case. However, the temporal relation between the vaccination and the onset of herpes zoster favored the possibility of vaccine-induced disease in our patient. Vaccine-induced lymphocytopenia is cited as a reason for herpes zoster following COVID-19 vaccination.[10] Psichogiou et al. proposed that vaccine-induced reactivation of herpes zoster could have similarities to immune reconstitution inflammatory syndrome, following antiretroviral treatment (unmasking of a pre-existing infection by the regained ability of the host, to mount an inflammatory response).[11] They postulated that following COVID-19 vaccination, varicella-zoster-specific CD8+ T lymphocytes become temporarily incapable of controlling the virus.[11] Although vaccination itself can induce a physical trauma (which is identified as a precipitating factor for reactivation of zoster virus), there are only a few reports of herpes zoster following different vaccines.[11] Triantafyllidis et al. have suggested to consider prophylaxis (for those at high risk for herpes zoster) with valacyclovir, before COVID-19 vaccination.[15]
We report this case to highlight the possibility of manifestation of herpes zoster following COVID-19 vaccination. The awareness regarding the higher risk of herpes zoster following COVID-19 vaccine may help the clinician to offer prompt diagnosis and effective management to the affected.
Declaration of patient consent
Not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- COVID-19 Commission of Accademia Nazionale dei Lincei, Rome, COVID-19 vaccines: Where we stand and challenges ahead. Cell Death Differ. 2021;28:626-39.
- [CrossRef] [PubMed] [Google Scholar]
- Cutaneous reactions reported after Moderna and Pfizer COVID-19 vaccination: A registry-based study of 414 cases. J Am Acad Dermatol. 2021;85:46-55.
- [CrossRef] [PubMed] [Google Scholar]
- Cutaneous adverse reactions after COVID-19 vaccines in a cohort of 2740 Italian subjects: An observational study. Dermatol Ther 2021:e15153.
- [CrossRef] [Google Scholar]
- Clinical and histopathological spectrum of delayed adverse cutaneous reactions following COVID-19 vaccination. J Cutan Pathol 2021
- [CrossRef] [PubMed] [Google Scholar]
- Erythema nodosum (EN), zoster duplex and pityriasis rosea as possible cutaneous adverse effects of Oxford-AstraZeneca COVID-19 vaccine: Report of three cases from India. J Eur Acad Dermatol Venereol 2021
- [CrossRef] [PubMed] [Google Scholar]
- World's Largest Vaccination Programme Begins in India on January 16. In: The Hindu. 2021.
- [Google Scholar]
- Post COVID-19 vaccination papulovesicular pityriasis rosea-like eruption in a young male. Dermatol Ther. 2021;34:e15040.
- [CrossRef] [PubMed] [Google Scholar]
- Herpes zoster after inactivated COVID-19 vaccine: A cutaneous adverse effect of the vaccine. In: J Cosmet Dermatol. 2021.
- [CrossRef] [PubMed] [Google Scholar]
- Recurrent herpes zoster after COVID-19 vaccination in patients with chronic urticaria being treated with cyclosporine-A report of 3 cases. J Cosmet Dermatol. 2021;1:1-3.
- [CrossRef] [PubMed] [Google Scholar]
- Herpes zoster after COVID vaccination. Int J Infect Dis. 2021;111:169-71.
- [CrossRef] [PubMed] [Google Scholar]
- Reactivation of varicella zoster virus after vaccination for SARS-CoV-2. Vaccines (Basel). 2021;9:572.
- [CrossRef] [PubMed] [Google Scholar]
- Risk factors for herpes zoster infection: A meta-analysis. Open Forum Infect Dis. 2020;7:ofaa005.
- [CrossRef] [PubMed] [Google Scholar]
- Zoster (Shingles) ACIP Vaccine Recommendations. 2019. Available from: https://www.cdc.gov/vaccines/hcp/acip-recs/vacc-specific/shingles.html [Last accessed on 2019 Feb 24]
- [Google Scholar]
- The epidemiology of herpes zoster in immunocompetent, unvaccinated adults ≥50 years old: Incidence, complications, hospitalization, mortality, and recurrence. J Infect Dis. 2020;222:798-806.
- [CrossRef] [PubMed] [Google Scholar]
- Varicella zoster virus reactivation following COVID-19 vaccination: A systematic review of case reports. Vaccines (Basel). 2021;9:1013.
- [CrossRef] [PubMed] [Google Scholar]





