Translate this page into:
Comparison between fine-needle aspiration cytology and histopathology in leprosy: A cross-sectional study
-
Received: ,
Accepted: ,
How to cite this article: Dominic S, Asokan N, Nandakumar G, George B. Comparison between fine-needle aspiration cytology and histopathology in leprosy: A cross-sectional study. J Skin Sex Transm Dis 2022;4:227-32.
Abstract
Objectives:
The objectives of the study were (1) to study the fine-needle aspiration cytology (FNAC) features of skin lesions of leprosy, (2) to determine the agreement between FNAC and histopathology to classify leprosy into different groups of the spectrum, and (3) to determine the sensitivity and specificity of FNAC to classify leprosy into different groups of the spectrum against the gold standard of histopathology.
Materials and Methods:
All newly diagnosed cases of leprosy who attended the outpatient department of dermatology and venereology of a tertiary referral center during the 16 months study period were included in this cross-sectional study. Based on FNAC and histopathology, patients were classified into different groups of the spectrum. Agreement between FNAC and histopathology to classify leprosy was determined by Kappa statistics. Sensitivity and specificity of FNAC to classify leprosy were determined against the gold standard of histopathology.
Results:
All the 47 study participants had histopathology features of leprosy. FNAC could obtain adequate aspirate in 30 patients (63.8%), who were considered for further analysis. There was moderate agreement (76.6%) between classification of leprosy by FNAC and histopathology on Kappa statistics (Kappa value 0.766). FNAC showed 80–100% sensitivity and 84–100% specificity to classify leprosy against the gold standard of histopathology.
Limitation:
Small sample size.
Conclusion:
When adequate aspirate is obtained, FNAC could serve as a useful tool in classification of leprosy.
Keywords
Leprosy
Fine-needle aspiration cytology
Histopathology
Agreement
Sensitivity
Specificity
INTRODUCTION
With the declared ‘elimination of leprosy,’ as a problem of public health importance, the current priority is to diagnose cases as early as possible and treat them with adequate fixed duration treatment so as to break the chain of transmission in the community. Diagnosis of leprosy is based on the cardinal criteria proposed by the World Health Organization (WHO).[1] Two of which are clinical criteria and the third depends on the skin smear findings.[1] Histopathology aids in proper classification of leprosy into different groups of the spectrum. An alternative easier option would be fine-needle aspiration cytology (FNAC). The first report of diagnosis and classification of leprosy by FNAC of skin lesions appeared in 1994.[2]
In FNAC, cytological features are studied in the smear prepared from the material obtained by needle aspiration. This simple office procedure can be done in an outpatient department and does not require local anesthesia. Moreover discomfort to the patient is minimum. Interpretation requires approximately 24 hours. The main drawback that has been cited against the wide spread application of FNAC is its low sensitivity since the analysis is carried out on limited material.[3]
This study was undertaken to document the FNAC findings of the skin lesions of leprosy and to compare the disease classification based on FNAC with the same based on histopathology.
MATERIALS AND METHODS
All newly diagnosed cases of leprosy (patients who satisfied the WHO cardinal criteria for leprosy) and who attended the outpatient department of dermatology and venereology of a tertiary referral center during the 16 months study period were included in this descriptive study after obtaining written informed consent.[1] Ethics committee of the institution approved the study. Patients diagnosed as pure neuritic leprosy and those aged less than 10 years were excluded from the study.
Using a pre-set proforma detailed history including the patient identification details, presenting complaints, duration of the disease, and family history of leprosy was collected. General examination and dermatological examination with special reference to morphology and distribution of skin lesions and testing for sensations were done. We did a detailed examination of peripheral nerves to detect nerve thickening and nerve function impairment.
FNAC [Figure 1] was done from the skin lesion using a 22 gauge needle and 10 ml disposable syringe under local aseptic precautions. A minimum of two smears were prepared from the material obtained from each patient. One smear was stained with modified Ziehl- Neelsen method to detect acid fast bacilli (AFB). Remaining smears were immediately placed in a jar containing isopropyl alcohol as fixative. Fixed smears were stained using Papanicolaou technique.

- Fine-needle aspiration of skin lesion of leprosy using a 22 gauge needle and 10 ml disposable syringe.
We did a skin biopsy (of the lesion from which FNAC was taken) under local anesthesia for histopathology analysis. Biopsies from the skin lesions were stained using hematoxylin and eosin to study the morphology and Wade-Fite technique to identify the AFB.
The patients were categorized into different groups of the spectrum of leprosy based on the cytological criteria proposed by Singh et al. and histopathology findings, respectively [Table 1].[2,4] Since FNAC does not distinguish between TT and BT, the terminology TL was used when the FNAC features were suggestive of TT or BT.[2]
| Leprosy spectrum | FNAC findings | Histology findings |
|---|---|---|
| Tuberculoid leprosy | Cellular smears showing cohesive epithelioid cell granulomas and numerous lymphocytes not infiltrating the granuloma with a bacteriological index of zero | Compact epithelioid granuloma with Langhan’s giant cells |
| Borderline tuberculoid leprosy | Cellular smears showing cohesive epithelioid cell granulomas and numerous lymphocytes not infiltrating the granuloma with a bacteriological index of zero | Epithelioid granuloma, not as compact as in tuberculoid spectrum, foreign body giant cells |
| Midborderline leprosy | Fair cellular aspirate showing poorly cohesive granulomas composed of epithelioid cells and macrophages. Presence of a few lymphocytes infiltrating the granuloma, BI 1+to 2+ | Diffuse epithelioid granuloma, no giant cells. BI 1+to 2+on Wade-Fite stain |
| Borderline lepromatous leprosy | Moderate cellularity showing singly dispersed macrophages with negative images, absence of epithelioid cells, numerous lymphocytes admixed with macrophages, BI 3+to 4+ | Macrophage granuloma with plenty of lymphocytes, Wade-Fite stain showing acid fast bacilli, BI 3+to 4+. |
| Lepromatous leprosy | Heavy cellularity with numerous foamy macrophages in a fatty back ground with numerous intracellular and extracellular negative images. Presence of a few lymphocytes, BI 5+to 6+ | Sheets of foamy macrophages with scanty lymphocytes. Wade-Fite stain showing acid fast bacilli with globi formation. BI 5+to 6+ |
WHO: World health organization, FNAC: Fine-needle aspiration cytology, BI: Bacteriological index
Data were analyzed using Statistical Package for the Social Sciences version 10. The data were expressed as frequencies and percentages. Agreement between, diagnosis based on FNAC and histopathology (gold standard) was determined by Kappa statistics. We also determined the sensitivity and specificity of FNAC to classify leprosy into different groups of the disease spectrum against the gold standard.
RESULTS
The study participants included 47 patients. The lesions aspirated included macules (10/47, 21.3%), plaques (29/47, 61.7%), and nodules (8/47, 17%). Aspirate was adequate for cytological studies in 30 (63.8%) patients. In 17 cases (36.2%), aspirate showed only blood. FNAC smears from most of the nodules (7/8, 87.5%) and majority of the plaques (20/29, 69%) showed adequate aspirate whereas those from most of the macules were inadequate (7/10, 70%). This difference in the yield of aspirate with the morphology of the lesion was found statistically significant (P = 0.03).
Among the 47 patients, four were TT [8.5%, Figure 2a], 18 BT (38.3%), one BB (2.1%), seven BL [14.9%, Figure 2b], 13 LL [27.7%, Figure 2c], and four (8.5%) indeterminate according to histopathology [Table 2]. The 30 patients whose FNAC gave adequate aspirate were classified into TL [TT/BT, 11–36.7%, Figure 3a], BB (1, 3.3%), BL [8, 26.7%, Figure 3b], LL [8, 26.7%, Figures 4a and b], and indeterminate leprosy [2, 6.7%, Figure 5]. FNAC could not differentiate between TT and BT [Table 2].
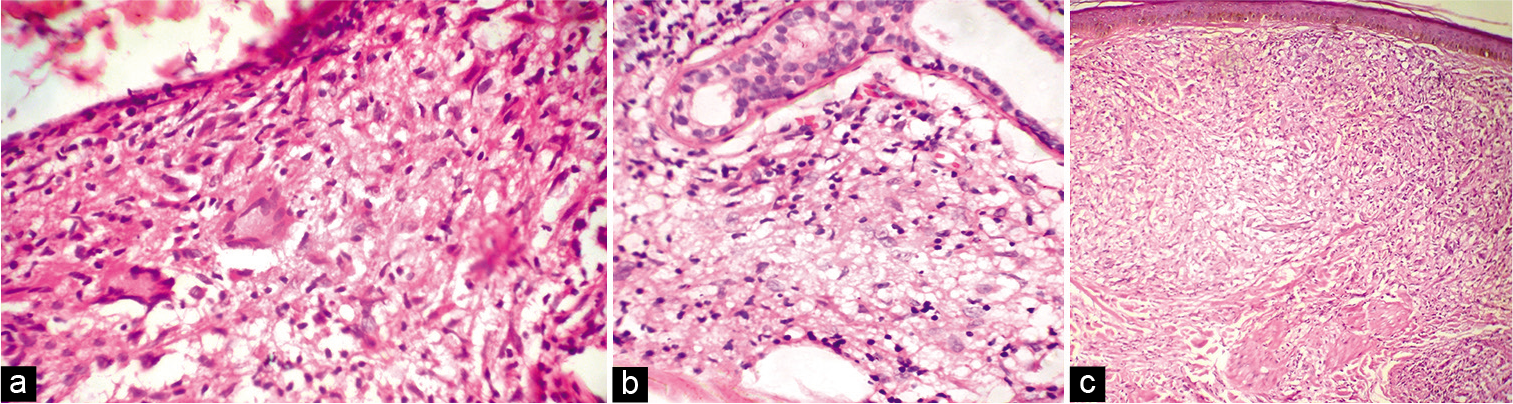
- (a) Skin biopsy from the lesion of tubeculoid leprosy showing epithelioid granuloma and Langhan’s giant cell (H and E, ×400); (b) Skin biopsy from the lesion of borderline lepromatous leprosy showing macrophage granuloma (H and E, ×400); (c) Skin biopsy from the lesion of lepromatous leprosy showing sheets of foamy macrophages (H and E, ×400).
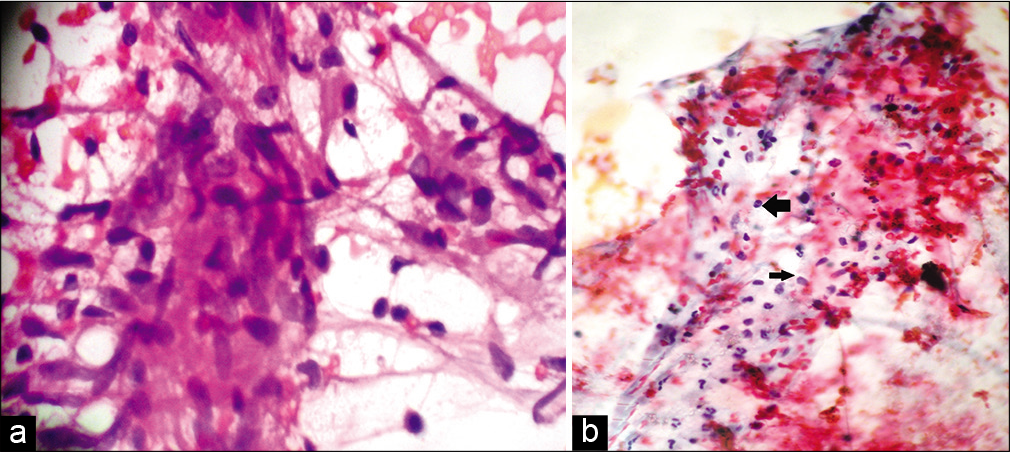
- (a) Fine-needle aspiration cytology smear from the lesion of tuberculoid leprosy showing cohesive epithelioid cell granulomas and numerous lymphocytes not infiltrating the granuloma (Papanicolaou stain, ×400); (b) Fine-needle aspiration cytology smear from the lesion of borderline lepromatous leprosy showing moderate cellularity and numerous lymphocytes (left arrow) admixed with macrophages (right arrow) (Papanicolaou stain, ×400).
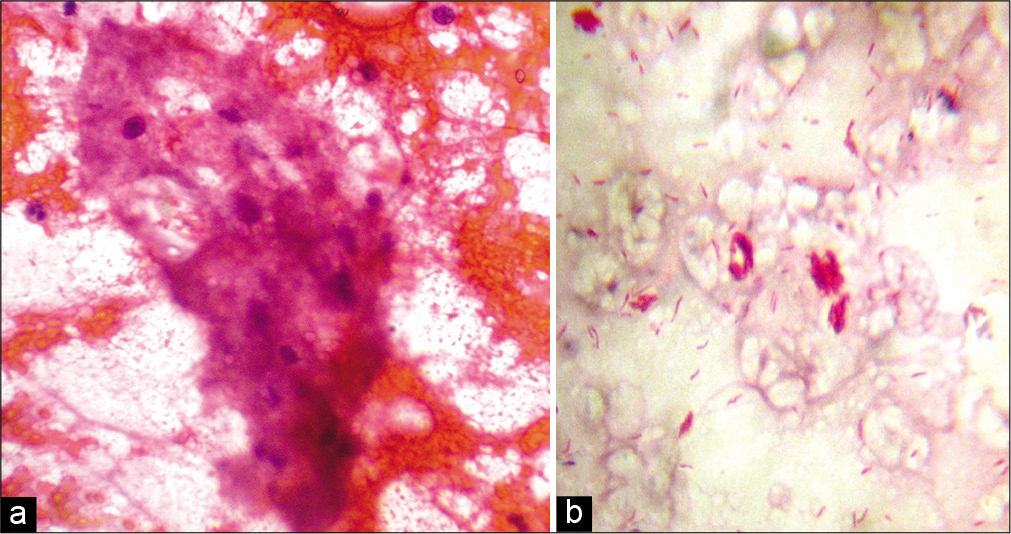
- (a) Fine-needle aspiration cytology smear from the lesion of lepromatous leprosy showing foamy macrophages in a fatty back ground and a few lymphocytes (Papanicolaou stain, ×400); (b) fine-needle aspiration cytology smear from the same lesion showing globi (Ziehl-Neelsen stain, ×1000).
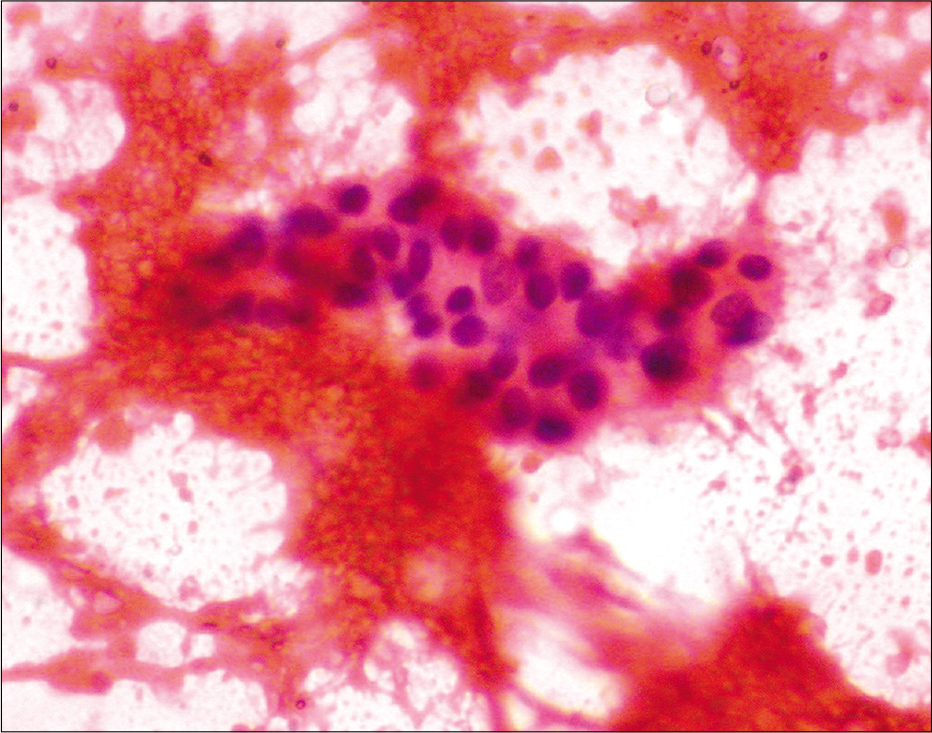
- Fine needle aspiration cytology smear from the lesion of indeterminate leprosy showing only lymphocytes (Papanicolaou stain, ×400).
| Spectrum of leprosy | *Number of patients whose FNAC had adequate aspirate(n=30, 100%) | *Number of patients whose histopathology confirmed leprosy (n=47, 100%) |
|---|---|---|
| Tuberculoid/Borderline tuberculoid leprosy | 11 (36.7) | 22 (46.8) |
| Midborderline leprosy | 1 (3.3) | 1 (2.1) |
| Borderline lepromatous leprosy | 8 (26.7) | 7 (14.9) |
| Lepromatous leprosy | 8 (26.7) | 13 (27.7) |
| Indeterminate leprosy | 2 (6.7) | 4 (8.5) |
Further analysis carried out in the 30 patients (whose FNAC had adequate aspirate), showed a moderate agreement (76.6%) between the classification based on FNAC and the same based on histopathology [Kappa value 0.766, Table 3].
| *Classification of leprosy based on histopathology | ||||||||
|---|---|---|---|---|---|---|---|---|
| Type of leprosy based on FNAC | TT | BT | BB | BL | LL | I | Total | Agreement between FNAC and histopathology |
| TL | 2 | 9 | 0 | 0 | 0 | 11 | Kappa value: 0.766 | |
| BB | 0 | 1 | 0 | 0 | 0 | 0 | 1 | |
| BL | 0 | 1 | 1 | 4 | 2 | 0 | 8 | |
| LL | 0 | 0 | 0 | 0 | 8 | 0 | 8 | |
| I | 0 | 0 | 0 | 0 | 0 | 2 | 2 | |
| Total | 2 | 11 | 1 | 4 | 10 | 2 | 30 | |
The sensitivity and specificity of FNAC to classify the 30 patients (in whom adequate aspirate was obtained on FNAC) into different groups of the spectrum against the gold standard of histopathology are shown in Table 4.
| Diagnosis based on histopathology (n = 30)* | Classification based on fine-needle aspiration cytology (n = 30) | Sensitivity of FNAC to classify leprosy | Specificity of FNAC to classify leprosy | ||||
|---|---|---|---|---|---|---|---|
| TL | BB | BL | LL | I | |||
| TL (n= 13) | 11 | 1 | 1 | 11/13 (84.6%) | 17/17 (100%) | ||
| BB (n = 1) | 1 | 0/1 (0%) | 28/29 (96.6%) | ||||
| BL (n = 4) | 4 | 4/4 (100%) | 22/26 (84.6%) | ||||
| LL (n = 10) | 2 | 8 | 8/10 (80%) | 20/20, (100%) | |||
| I (n = 2) | 2 | 2/2 (100%) | 28/28 (100%) | ||||
*Only the thirty patients whose skin lesions yielded adequate aspirate on fine-needle aspiration are included. FNAC: Fine-needle aspiration cytology, TL: Tuberculoid/ Borderline tuberculoid leprosy, BB: Midborderline leprosy, BL: Borderline lepromatous leprosy, LL: Lepromatous leprosy, I: Indeterminate leprosy
DISCUSSION
We found that FNAC could obtain adequate aspirate to give a disease classification in only 63.8% of leprosy patients. FNAC showed less chance of procuring adequate aspirate from macular skin lesions in comparison to plaques and nodules.
FNAC showed moderate agreement (Kappa value 0.766) with the gold standard in classifying leprosy into different groups of the spectrum. When adequate aspirate was obtained, FNAC showed 80–100% sensitivity to classify leprosy patients. Interestingly two patients diagnosed as BT on histopathology were classified as BB and BL, respectively, on FNAC. This underscores the importance of WHO recommendation that the decision on whether to give paucibacillary or multibacillary antileprosy treatment should be based on the number of skin lesions and the peripheral nerves involved and the skin smear status, rather than on the histopathology diagnosis.[1]
The predominant clinical types of leprosy observed by us (BT, LL, and BL) in the study population were consistent with several previous studies.[4-6]
We obtained adequate aspirate on FNAC in 63.8% of cases, which was similar to the observation of Rao et al. (64.9%).[5] The previous authors found macules as the lesions giving lowest yield of aspirate on fine needle aspiration, which was comparable to our observations.[2,5]
Rao et al. could classify FNAC smears into only three groups which included tuberculoid, borderline, and lepromatous types.[5] We could classify the cytology findings in all the smears with adequate aspirate into different groups (TL, BB, BL, and LL) as proposed by Singh et al.[2] The distribution of patients according to cytological classification by us was consistent with the studies by Nigam et al. and Prasad et al.[4,7]
We classified smears having only lymphocytes as indeterminate type. This type was not described under the cytological criteria by Singh et al.[2] Even though Nigam et al. included indeterminate type in their cytohistological correlative study, they have not mentioned the specific cytological features of indeterminate type.[4]
We failed to detect any “intracellular or extracellular negative images” which corresponded to M. leprae in acid fast staining as described in various studies.[2,4,5,8] This could be due to the difference in the stain used in our study. We used Papanicolaou technique while intracellular or extracellular negative images were noted when the aspirate was stained with May-Grunwald Giemsa stain.[2,4,5,8]
We observed moderate to good cellularity in TL and LL whereas there was limited number of cells in the smears which turned out to be indeterminate type according to cytological features. The previous studies also showed a similar finding in the case of TL and LL types but there were no studies commenting about the amount of cells in FNAC smears which showed features of indeterminate type.[4,5,7] The poor cellularity in indeterminate leprosy is probably a reflection of a lesser degree of inflammatory infiltrate in the lesions.
We used Kappa statistics to determine the agreement between the disease classification based on FNAC and histopathology. However, most of the previous studies had used the percentage correlation which does not allow assessment of statistical significance. Comparison with previous studies is handicapped by this fact.[2,5]
The FNAC’s ability to obtain adequate aspirate from 63.8% of lesions as observed by us was comparable to the 64.9% reported by Rao et al.[5] However, all the cases, where we got adequate aspirates (63.8%), could be classified based on the cytological features. Rao et al. could get adequate aspirates in 64.9% patients, but could classify only 65.5% of them based on the cytological features.[5] In the study by Singh et al., 76.7% aspirates were adequate and they were able to classify all the cases based on cytology.[2]
We observed moderate agreement between FNAC based classification of leprosy cases and the classification based on histopathology (Kappa value 0.766).[2,5]
The sensitivity of FNAC to classify the patients (in whom adequate aspirate was obtained) into different groups varied from 80% to 100%. FNAC was unable to differentiate BT from TT. FNAC showed 84.6–100% specificity to classify patients into different groups of the spectrum. More importantly, FNAC classified all the 14 patients with BL/LL in whom adequate aspirate was obtained in the lepromatous group of the spectrum. This assumes significance since, though rare, single lesion lepromatous cases are reported.[9] An accurate differentiation from tuberculoid disease is essential to ensure adequate treatment in such instances.
Limitation
Small sample size was a limitation of our study. Using a dry syringe for obtaining aspirate from skin lesions for cytology analysis might have compromised the yield of aspirate in our study.
CONCLUSION
Although FNAC may have only a limited role in diagnosis of leprosy, it is a useful tool in classifying leprosy into different groups of the spectrum, if adequate aspirate is obtained.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
Dr N Asokan, Dr G Nandakumar and Dr Biju George are on the editorial board of the Journal.
References
- Guidelines for the Diagnosis. 2019. Treatment and Prevention of Leprosy. Executive Summary. Geneva: World Health Organization; Available from: http://www.searo.who.int/entity/global_leprosy_programme/approved-guidelines-leprosy-executives-summary [Last accessed on 2019 Jun 19]
- [Google Scholar]
- Nodular lepromatous leprosy: Report of a case diagnosed by FNA. Diagn Cytopathol. 1994;11:373-5.
- [CrossRef] [PubMed] [Google Scholar]
- Manual and Atlas of Fine Needle Aspiration Cytology (3rd ed). Edinburg: Churchill Livingstone; 1999.
- [Google Scholar]
- Fine needle aspiration cytology in reactional and non-reactional leprosy. Indian J Dermatol Venereol. 2007;73:247-9.
- [CrossRef] [PubMed] [Google Scholar]
- Utility of fine-needle aspiration cytology in the classification of leprosy. Diagn Cytopathol. 2001;24:317-21.
- [CrossRef] [PubMed] [Google Scholar]
- Are children safe from complications of leprosy? A study from North Kerala. J Skin Sex Transm Dis. 2020;2:31-4.
- [CrossRef] [Google Scholar]
- Fine needle aspiration cytology in leprosy. Indian J Dermatol Venereol Leprol. 2008;74:352-6.
- [CrossRef] [PubMed] [Google Scholar]
- Negative images in the fine needle aspiration cytologic diagnosis of mycobacterial infections. Malays J Pathol. 2001;23:89-92.
- [Google Scholar]
- A single skin lesion-an unusual presentation of lepromatous leprosy. Int J Lepr Other Mycobact Dis. 1985;53:554-8.
- [Google Scholar]






