Translate this page into:
A clinical study of idiopathic guttate hypomelanosis and its association with diabetes mellitus
*Corresponding author: S. Pradeep Nair, Department of Dermatology and Venereology, Government Medical College, Trivandrum, Kerala, India. dvmchtvm@yahoo.co.in
-
Received: ,
Accepted: ,
How to cite this article: Nair SP, Sreenivas B. A clinical study of idiopathic guttate hypomelanosis and its association with diabetes mellitus. J Skin Sex Transm Dis 2021;3:162-5.
Abstract
Objectives:
To study the clinical profile of idiopathic guttate hypomelanosis (IGH) and its association with diabetes mellitus (DM).
Materials and Methods:
A 1-year descriptive study was carried out in clinically diagnosed cases of IGH. A detailed history was taken and a thorough dermatological examination was carried out in each case. Serum fasting blood sugar [FBS] and postprandial blood sugar (PPBS) levels were determined in all cases. FBS and PPBS were assessed in age- and sex-matched individuals without IGH who were included as the comparative group.
Results:
There were a total of 102 patients (n = 102) with IGH in the designated study period. The male/female ratio was 1:3.08. The mean age was 36.7 years. The most common age group was 61–70 years (29, 28.4%). History of chronic sun exposure was present in 19 (18.6%). Thirty-one patients (30.4%) showed lesions affecting upper limb, lower limb, and trunk. In the present study, 23 patients with IGH had DM. In the age- and sex-matched comparative group, 19 patients had DM (18.6%). The difference was not statistically significant (P = 0.498). In the present study, 17 patients (17/23, 73.9%) with IGH and DM had 20 lesions or more, while 6 patients (6/23, 26.1%) with IGH and DM had < 20 lesions. Among the 79 non-diabetic patients with IGH, 57 (72.2%) had 20 lesions or more and 22 (27.8%) had less than 20 lesions. This was not statistically significant (P = 0.868).
Limitations:
Small sample size was the major limitation.
Conclusion:
There was no association between IGH and DM in this study and there was no association between number of lesions of IGH and DM.
Keywords
Idiopathic guttate hypomelanosis
Diabetes mellitus
Sun exposure
INTRODUCTION
Idiopathic guttate hypomelanosis (IGH) is a common acquired hypomelanotic disorder that usually occurs after 40 years of age and is more common in females. It usually occurs in the sun exposed areas even though covered sites like trunk can be affected.[1] IGH manifests as multiple, discrete, hypopigmented to depigmented, guttate macules, that vary in diameter from 0.5-6 mm [Figure 1].[2] The lesions are asymptomatic and are often only of cosmetic concern. No treatment is required and there is no definite treatment. Cryotherapy and dermabrasion may have some benefit. Diabetes mellitus (DM) is a metabolic disorder with a broad spectrum of cutaneous manifestations. IGH has been described as a non-specific cutaneous manifestation of DM.[3] It may sometimes precede DM. Studies denoting association between IGH and DM are limited. This study focuses on the clinical profile of IGH and its association with DM.
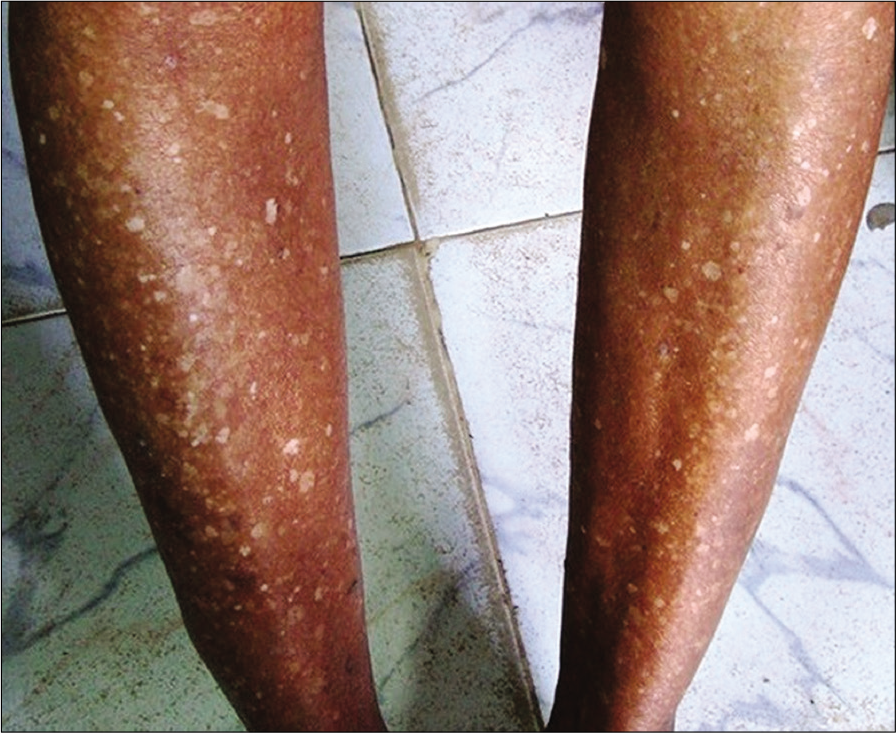
- Hypopigmented macules of idiopathic guttate hypomelanosis in the lower limbs.
MATERIALS AND METHODS
This is a 1-year descriptive study done in a tertiary care center. The sample population included all clinically diagnosed cases of IGH in the study period attending the outpatient clinic. After getting written informed consent from individual study participant, we collected detailed history and carried out a thorough dermatological examination. The duration, gender, age, and occupation were the main demographic data recorded. Each study participant was specifically asked about personal or family history of DM and family history of IGH. We noted the size, number and distribution of lesions of IGH.
We evaluated serum fasting blood sugar (FBS) level after overnight fasting of 12 h and postprandial blood sugar (PPBS) level, 1 ½ h after meals, in all patients. The standard values taken were FBS 126 mg% and PPBS 200 mg% as per American Diabetic Association.[4]
Age- and sex-matched individuals without IGH were taken as the comparative group. After written informed consent, FBS and PPBS levels of controls were assessed.
The data collected were analyzed in terms of mean, frequency, and percentage. The Chi-squared test was applied to note if there was any statistically significant difference between the study group and the comparative group and P < 0.05 was considered as statistically significant.
Permission to conduct this study was granted by the Institutional Ethics Committee of this tertiary care center.
RESULTS
There were a total of 102 patients (n = 102) with IGH in the designated study period. An equal number (n = 102) of ageand sex-matched individuals were taken for the comparative group. The demographic details of the study group are given in Table 1. The male/female ratio was 1:3.08. The age of the patients ranged from 15 to 78 years. The mean age was 36.7 years. The most common age group was 61–70 years (29, 28.4%). Majority of the study participants were home makers (56, 54.9%). The salient clinical features are given in Table 2. The history of DM and family history details are given in Table 3. The dermatological findings are given in Table 4. In the present study, 23 patients (22.5%) had history of DM. Fifteen (14.7%) were on oral hypoglycemic agents, 1 (0.98%) on insulin, and 7 (6.9%) were on diet control only. At the time of examination, 8 patients (7.8%) had FBS >126 mg% and 7 (6.9%) patients had PPBS >200 mg%. In the ageand sex-matched comparative group, 19 patients (18.6%) had DM [Figure 2]. This was not statistically significant (P = 0.498). Seventeen patients (17/23, 73.9%) with IGH and DM had 20 lesions or more, while six patients (6/23. 26.1%) with IGH and DM had < 20 lesions. Among the 79 non-diabetic patients with IGH, 57 (72.2%) had 20 lesions or more and 22 (27.8%) had less than 20 lesions. This was also not statistically significant (P = 0.868).
| Demographic feature | Frequency (percentage) |
|---|---|
| Gender | |
| Male | 25 (24.5) |
| Female | 77 (75.5) |
| Age group in years | |
| <20 | 2 (2) |
| 21–30 | 2 (2) |
| 31–40 | 9 (8.8) |
| 41–50 | 26 (25.5) |
| 51–60 | 26 (25.5) |
| 61–70 | 29 (28.4) |
| >70 | 8 (7.8) |
| Occupation | |
| Unemployed | 4 (3.9) |
| Manual laborer | 6 (5.9) |
| Skilled | 11 (10.8) |
| Business | 5 (4.9) |
| Homemaker | 56 (54.9) |
| Others | 20 (19.6) |
| Clinical features | Frequency (percentage) |
|---|---|
| Age of onset in years | |
| <20 | 3 (2.9) |
| 21–30 | 2 (2) |
| 31–40 | 21 (20.6) |
| 41–50 | 21 (20.6) |
| 51–60 | 32 (31.4) |
| 61–70 | 20 (19.6) |
| >70 | 3 (2.9) |
| Duration in years | |
| <5 | 73 (71.6) |
| 6–10 | 24 (23.5) |
| 11–15 | 2 (2) |
| 16–20 | 2 (2) |
| >20 | 1 (1) |
| Chronic sun exposure | |
| Present | 19 (18.6) |
| Absent | 83 (81.4) |
| Diabetic status | Frequency (percentage) |
|---|---|
| DM in person | |
| Present | 23 (22.5) |
| Absent | 79 (77.5) |
| Duration of DM (years) | |
| <10 | 15 (65.3) |
| 11–20 | 7 (30.4) |
| 21–30 | 1 (4.4) |
| Family history of DM | |
| Present | 32 (31.4) |
| Absent | 70 (68.6) |
| Family history of IGH | |
| Present | 19 (18.6) |
| Absent | 83 (81.4) |
DM: Diabetes mellitus, IGH: Idiopathic guttate hypomelanosis
| Lesions of idiopathic guttate hypomelanosis | Frequency (percentage) |
|---|---|
| Number of lesions | |
| 1–5 | 4 (3.9) |
| 6–20 | 24 (23.5) |
| >20 | 74 (72.5) |
| Size of the lesions in mm | |
| 1–5 | 75 (73.5) |
| 6–10 | 26 (25.5) |
| >10 | 1 (1) |
| Distribution of lesions | |
| Upper limb | 4 (3.9) |
| Lower limb | 24 (23.5) |
| Upper limb, lower limb, and trunk | 31 (30.4) |
| Upper limb and trunk | 4 (3.9) |
| Lower limb and trunk | 9 (8.8) |
| Upper and lower limbs | 30 (29.4) |
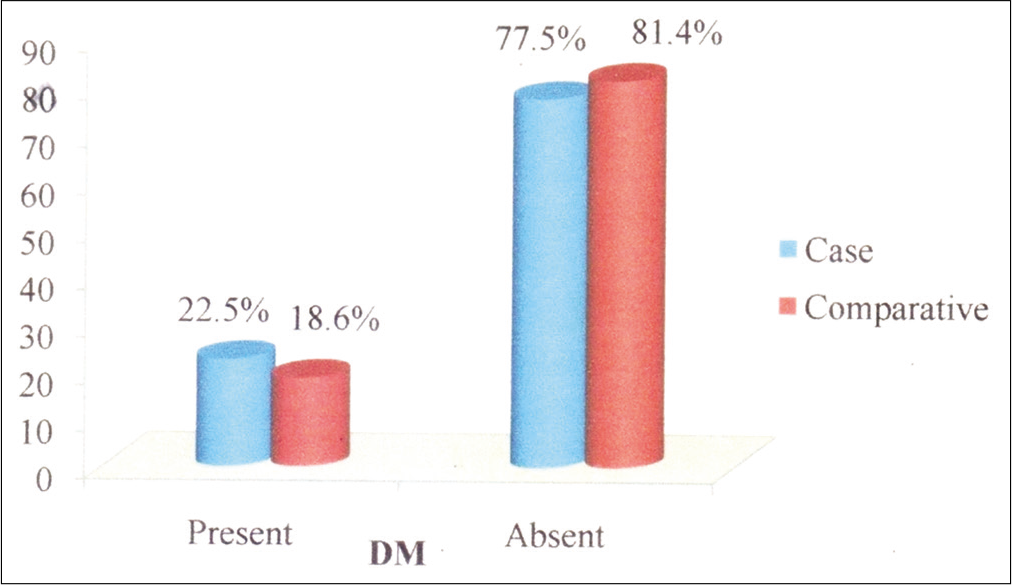
- Chart showing comparison of diabetes mellitus in idiopathic guttate hypomelanosis patients and in the comparative group.
DISCUSSION
A female predominance (75.5%) was noted in this study. This is similar to the study conducted by Falabella and Whitehead et al.[5,6] Since sunlight exposure is considered to be one etiological factor, we expect males to be affected more due to their greater outdoor activity. This again adds to the fact that the etiology of IGH is unclear. In this study, IGH was most commonly seen in the advancing age group of 61–70 years (28.4%). This is similar to the study done by Sahoo et al. and Kim et al.[7,8] Thus, IGH is a disease of advancing age. With advancing age, the number and size of lesions also increase. Age-related somatic mutation has been suggested as the pathological mechanism.[9] Majority of the patients in this study were homemakers (54.9%), again indicating that sunlight may not play a role in the pathogenesis of IGH. Another fact may be that females are more cosmetically concerned and may seek medical help early. History of chronic sun exposure was present in only 18.6%. The role of chronic sun exposure in causing IGH is not supported by most studies. Only the studies by Mollet et al. and Whitehead et al. showed a relation between chronic sun exposure and IGH.[5,10] Other studies did not show this relation, and on the contrary, non-actinic IGH lesions were commonly seen in non-exposed skin in Black Africans. [11,12] In the present study, 22.5% gave a history of DM. In a study by Regunatha et al, only 14.4% of 500 consecutive DM patients had IGH.[3,13] Hence, the present study and other studies in literature favor the lack of association between IGH and DM. Only 18.6% of patients in this study gave a family history of IGH. Some studies in literature have showed an increased prevalence of IGH in family members. [13] Although family history is seen in some studies, a strong family association was not established.
In the present study, majority of the patients (72.5%) were having greater than 20 lesions. This can be explained by the fact that most of the people in this study belonged to the elderly age group and it is a well-known fact that IGH increases with advancing age. Other studies have also corroborated this fact.[14] About 73.5% of the patients in this study had lesions of diameter 1–5 mm. Literature mentions that the size and number of IGH increase with the duration of the disease. The average duration of disease in this study was below 5 years, so this could explain the smaller lesions in this study. In this study, majority (30.4%) of the patients had IGH lesions involving multiple sites (trunk, upper limbs and lower limbs). Other studies reported majority of lesions in the lower limbs.[2,5,15] In this study, 23 patients in the study group (22.5%) had DM, while 19 subjects (18.6%) in the comparative group had DM, which was not statistically significant (P = 0.489). We found no statistically significant difference between diabetics and those without DM with IGH with respect to number of lesions. Therefore, this study did not show any association between IGH and DM or between number of lesions of IGH and DM.
Limitations of the study
Sample size is small.
CONCLUSION
This study did not show any relation between IGH and sunlight exposure. Multiple sites were most commonly involved. There was no association between IGH and DM and there was no association between number of lesions of IGH and DM. Future studies with larger sample size may further bring out more information regarding IGH and DM.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
Dr. S. Pradeep Nair is on the editorial board of the Journal.
References
- Clinical features of idiopathic guttate hypomelanosis in 646 subjects and association with other aspects of photoaging. Int J Dermatol. 2011;50:798-805.
- [CrossRef] [PubMed] [Google Scholar]
- Cutaneous disorders in 500 diabetic patients attending diabetic clinic. Indian J Dermatol. 2011;56:160-4.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnosis and classification of diabetes mellitus. Diabetes Care. 2011;34(Suppl 1):S62-9.
- [CrossRef] [PubMed] [Google Scholar]
- Idiopathic guttate hypomelanosis. Arch Dermatol. 1966;94:279-81.
- [CrossRef] [PubMed] [Google Scholar]
- Comprehensive understanding of idiopathic guttate hypomelanosis: Clinical and histopathological correlation. Int J Dermatol. 2010;49:162-6.
- [CrossRef] [PubMed] [Google Scholar]
- On the pathogenesis of idiopathic guttate hypomelanosis. J Am Acad Dermatol. 1987;16:34-44.
- [CrossRef] [Google Scholar]
- Origin, clinical presentation, and diagnosis of hypomelanotic skin disorders. Dermatol Clin. 2007;25:363-71.
- [CrossRef] [PubMed] [Google Scholar]
- Hypopigmented macules of photodamaged skin and their treatment with topical tretinoin. Acta Derm Venereol. 1999;79:305-10.
- [CrossRef] [PubMed] [Google Scholar]
- Pigmentary skin disorders in black Africans of Sierra Leon. J Pak Assoc Dermatol. 2007;17:4-10.
- [Google Scholar]
- Pigmentary disorders in Latin America. Dermatol Clin. 2007;25:419-30.
- [CrossRef] [PubMed] [Google Scholar]
- A clinical study of skin changes in geriatric population. Indian J Dermatol Venereol Leprol. 2009;75:305-6.
- [CrossRef] [PubMed] [Google Scholar]
- Idiopathic guttate hypomelanosis. Br J Dermatol. 1980;103:635-42.
- [CrossRef] [PubMed] [Google Scholar]






