Translate this page into:
Secondary lymphedema: Pathogenesis
*Corresponding author: Smitha Ancy Varghese, Dermatology and Venereology, Government Medical College, Thiruvananthapuram, Kerala, India. smitharijo@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Varghese SA. Secondary lymphedema: Pathogenesis. J Skin Sex Transm Dis 2021;3(1):7-15.
Abstract
Secondary lymphedema follows an acquired defect in the lymphatic system. The common causes leading to a defective lymphatic function include infection, inflammation, malignancy, trauma, obesity, immobility, and therapeutic interventions. Understanding the pathogenesis of lymphedema is of prime importance in offering effective treatment. The pathogenetic mechanisms such as lymphatic valvular insufficiency, obliteration/ disruption of lymphatic vessels, and decreased lymphatic contractility aggravate lymphatic hypertension and lymphstasis. Accumulation of lymph, interstitial fluid, proteins, and glycosaminoglycans within the skin and subcutaneous tissue eventually stimulates collagen production by fibroblasts, causes disruption of elastic fibers, and activates keratinocytes, fibroblasts, and adipocytes. These result in thickening of skin and cause fibrosis of subcutaneous tissue. However, the sequence of these pathomechanisms, their inter-relationship and progression vary depending on the specific etiology of the lymphedema. In this article, we discuss the possible cellular and molecular mechanisms involved in the pathogenesis. Further studies to delineate the exact sequence of pathogenic processes surrounding the primary triggering event can help to formulate tailored therapeutic approaches.
Keywords
Inflammation
Pathogenesis
Secondary lymphedema
INTRODUCTION
The lymphatic system plays two important functions in the human body. It maintains the fluid balance of the body by returning the protein deposits and extra tissue fluid extravasated from the blood capillaries to the circulation system. Besides, the lymphatic vessels carry germs and pathogens to the lymph nodes so that the immunological defense mechanism is activated.
Understanding the normal anatomy of the lymphatic system is crucial in comprehending the pathogenesis of lymphedema.[1]
The lymphatic vessels are categorized into lymph capillaries, pre-collectors, and lymph-collecting vessels. The lymph capillaries have a diameter ranging from 20 μm to 70 μm. These superficially located vessels are placed immediately beneath the epidermis. Lymph capillaries are formed by endothelial cells that connect loosely with each other like roof tiles (in an overlapping pattern). They lack valves. A fibrous anchoring filament joins the endothelial cell with the surrounding tissue. In the presence of excess interstitial fluid (edema), the junctions between the endothelial cells open up. This is achieved by the action of the anchoring filaments that pull the endothelial cells outward to capture the edema fluid into the lumen.
The capillaries join larger pre-collectors (70–150 μm in diameter) in the deep dermis. Pre-collectors through their valvular structure permit lymph flow in one direction only (from the superficial to the deep layers). Pre-collectors join together within the dermis to form larger vessels called efferent pre-collectors that run vertically through the subcutaneous tissue.
The efferent pre-collectors connect to collectors (the lymph collecting vessels of 150–500 μm in diameter) in the subcutaneous fat layer. The collectors are oriented horizontally in the subcutaneous tissue and have a triple-layered wall of endothelial cells, smooth muscle cells, and collagen fibers with fibroblasts. The rhythmic contraction of fibroblasts propels the lymph flow. The collectors are further sub-classified into superficial and deep vessels based on their anatomical relationship to the deep fascia. The deep vessels follow the arteries, while superficial vessels show no such preference.[1]
Each lymphatic vessel connects to at least one lymph node before joining the vein. This ensures that pathogens or cancer cells are not released into the systemic blood circulation before the lymph nodes activate the immune system.[1]
In 2018, Suami and Scaglioni introduced the concept of ‘lymphosome’ that suggested that the lymphatic vessels in a particular region connect to the same subgroup of regional lymph nodes.[1]
Peripheral edema is an outcome of lymphatic failure which can either be relative or absolute. Relative lymphatic failure occurs when microvascular filtration overwhelms the lymph drainage. The term “lymphedema” is reserved for absolute lymphatic failure (edema resulting principally from a failure in lymph drainage).[2]
Primary lymphedema refers to lymphedema due to congenital anomalies of lymphatics while secondary lymphedema arises from dysfunction of lymphatics due to acquired causes.[2]
The acquired causes for lymphedema range from infections and trauma to malignancies and surgeries. Hence, not surprisingly, secondary lymphedema is far more common than primary lymphedema and generally develops at a later age when compared to primary lymphedema.[2] With the progress made in genetics and, immunology and newer insights into the anatomy and physiology of the lymphatic system, the pathogenetic mechanisms have been elucidated with more clarity in recent times. This article will focus on the pathophysiological mechanisms of secondary lymphedema.
Pathophysiology of secondary lymphedema varies depending on the underlying conditions [Table 1]. Hence, the pathogenesis is better discussed under the subheadings of individual conditions causing secondary lymphedema.
| Causes | Pathogenesis |
|---|---|
| Infections | |
| Filariasis | • Pro-inflammatory milieu and injury to lymphatic vessel • Anatomic and physiologic alterations: Lymphangiectasia, granulomatous responses, collateral formation and decreased trans-endothelial transport • Promotion of lymphangiogenesis • Secondary bacterial infection and further damage |
| Cellulitis/erysipelas | • Further damage to the lymphatics by attendant inflammation • Local immunodeficiency and reactivation of infection |
| Tuberculosis, Lymphogranuloma venereum | • Inflammation of draining lymph nodes impeding the lymph flow |
| Malignant disease | |
| Large tumors | •Pressure effect on lymphatic vessels |
| Metastases | • Overexpression of lymphangiogenic growth factors: • Induce lymphatic endothelium to produce chemokine ligand 21 that attracts tumor cells to lymphatics • Increase lymph flow and tumor dissemination by increasing contractility of proximal collecting lymphatic vessels • Tumor growth in lymph nodes/vessels causes increased flow resistance. • Tumors lack functional lymphatic vessels within, leading to increased interstitial pressure |
| Treatment for malignancy | • Complete excision of a lymph node basin: Disrupts the normal return of lymphatic fluid •Radiation: • Fibrosis of surrounding tissue compresses and blocks the lymphatic flow, • Inhibition of proliferation of lymphatic vessels prevents compensatory growth of lymphatic vessels • Fibrosis of lymph nodes alters their ability to filter lymphatic fluid |
| Trauma and tissue damage | |
| Lymph node excision, burns, scarring, varicose vein surgery/vein harvesting, large wounds | • Damage to lymphatic vessels compromising the lymph flow |
| Venous disease | |
| Chronic venous insufficiency, venous ulceration, post-thrombotic syndrome, intravenous drug use | • Obliteration of parts of the lymphatic superficial capillary network • Cutaneous reflux of lymph from deep to superficial channels • Increased lymphatic capillary permeability • Collapse of lumen of intradermal lymphatics, loss of the open intercellular junctions, and damage to the anchoring filaments leading to impaired lymphatic function |
| Inflammation | |
| Rheumatoid arthritis, dermatoses including epidermal dermatoses, psoriasis, sarcoidosis | • Inflammatory mediators, such as nitric oxide and prostaglandins slow down the frequency of the contractions in lymphatic vessels, in turn, slowing down the lymph flow • Cytokines such as tumor necrosis factor-a and interleukin-1 mediate lymphatic dysfunction |
| Obesity | • External compression of lymphatics by adipose tissues • Increased production of lymph • Direct injury to the lymphatic endothelium by changes in body weight or diet • Lymphedema-associated fat deposition which is chronically inflamed and infiltrated by macrophages and lymphocytes • Increased production of inflammatory cytokines in obese |
| Immobility | • Lack of muscle activity (that usually massages fluid into and along lymphatic vessels) leading to stagnation of lymph flow |
| Pretibial myxedema | • Obstruction of dermal lymphatic vessels by mucin |
| Factitious | • Lymphatic compromise from prolonged pressure |
| Podoconiosis | • Immune response to soil antigen/mineral (aluminum, silicon, magnesium and iron) • Subendothelial edema and collagenization of afferent lymphatics leading to narrowing and eventual obliteration of the lumen |
INFECTIONS
In addition to filariasis (the most common cause of secondary lymphedema), the other infections which can lead to lymphedema are cellulitis/erysipelas, tuberculous lymphadenitis, and lymphogranuloma venereum.
Filariasis
The adult forms of filarial worms (Wuchereria bancrofti, Brugia malayi, and Brugia timori) living in the afferent lymphatics and/or the lymph nodes and their larval progeny (the microfilariae, that circulate in the peripheral blood where they infect mosquito vectors when they feed) contribute to the pathogenesis of filarial lymphedema.[3] A complex interplay of immunologic factors, endothelial factors, genetic factors, and superadded bacterial infections determines the outcome.
Immunologic factors
The lymphatic vessel injury and subsequent inflammatory response are attributed to specific pro-inflammatory cytokines such as tumor necrosis factor (TNF)-α, interleukin (IL)-6, and soluble TNF receptor.[4] Endothelin-1, IL-2, IL-8, macrophage inflammatory protein (MIP)-1α, MIP-1β, macrophage chemotactic protein-1, thymus and activation-regulated chemokine, and interferon-inducible protein-10 in the peripheral circulation also play a role.[5,6] A pro-inflammatory milieu exists within the lymphatic vessels, with elevated levels of gamma-globulins, α-1 acid glycoprotein, and IL-1β in the lymph fluid.[5]
Endothelial factors
Live filarial parasites and their secretory products play an important role in the early stage of the disease by inducing activation, proliferation, and tube formation by lymphatic endothelial cells. The serum from patients with filariasis has shown the presence of factors that promote significant lymphatic endothelial cell proliferation. Active lymphatic remodeling involving endothelial cell growth, migration, and proliferation leads to anatomical changes in the architecture of lymphatics ranging from lymphangiectasia and granulomatous responses to collateral formation.[7,8]
Genetic factors
The filarial parasites induce alterations in the normal physiology of the lymphatic endothelium. Global gene expression analysis revealed alterations in genes involved in junction adherence pathways that, in turn, decrease trans-endothelial transport. A role is proposed for the vascular endothelial growth factor (VEGF) family in lymphangiogenesis.[9,10] Other angiogenic factors such as angiopoietins-1 and 2 are also elevated in individuals with filariasis.
Superadded infections
The damaged lymphatics with leaky lymphatic endothelium act as a potential nidus for bacterial translocation. The affected lymphatics show increased bacterial and fungal loads.[3]
CELLULITIS/ERYSIPELAS
Lymphedema is an important predisposing factor for cellulitis. The protein-rich lymphatic fluid serves as an excellent medium for the bacteria to grow. Besides, stagnation of the lymphatic fluid due to impaired lymph drainage and consequent reduction in lymphatic clearance creates a state of immune deficiency locally, which, in turn, increases the risk of cellulitis. Cellulitis and lymphedema appear to have a reciprocal relationship – a pre-existing lymphatic defect predisposes to cellulitis and episodes of cellulitis further damage the lymphatic system. This vicious cycle is independent of the primary etiology of lymphedema.[10] Mortimer et al. suggested that once the bacteria have gained entry to edematous tissue, eradication proves difficult and there exists the risk of reactivation of cellulitis since the local immune system is impaired.[11]
TUBERCULOSIS/LYMPHOGRANULOMA VENEREUM (LGV)
Elephantiasis associated with tuberculosis and LGV closely resemble each other in their association with inguinal lymphadenitis. Tuberculosis can also produce pseudoelephantiasis (i.e., elephantiasis of genitalia secondary to genital pathology) with a similar clinical presentation.[12,13]
LYMPHEDEMA SECONDARY TO MALIGNANCY
Lymphatic metastasis and subsequent functional impairment of lymph channels leading to lymphedema are now considered as multi-step processes that include:
Attraction and entry of cancer cells into lymphatic vessels
In mouse models, the growth of lymph vessels in peripheral tumors is enhanced by over-expression of lymphangiogenic growth factors VEGF-C and VEGF which, in turn, increase the risk of lymph node metastasis. VEGF-C also enhances the production of chemokine ligand 21 by the lymphatic endothelium, thereby promoting the entry of chemokine receptor 7+ tumor cells into lymphatics.[14,15] Thus, tumor cells that reach the lymphatics may enter either passively or through the action of active signaling mechanisms.
Cancer cells travel through the collecting lymphatic vessels to the lymph node
Tumor-derived VEGF-C and VEGF-D increase the contraction of proximal collecting lymphatic vessels, thus potentially increasing the lymph flow and the dissemination of tumor cells.[16] As tumors grow in lymphatic vessels or overtake a lymph node, flow resistance increases and lymph is diverted around these structures through collateral lymphatic vessels.
Immunological changes in lymph node microenvironment
The immune cell populations in tumor-draining lymph nodes are altered. Several cytokines play a prominent role in producing immunosuppression of tumor-draining lymph nodes. The levels of IL-10, transforming growth factor-β, and granulocyte-macrophage colony-stimulating factor are all elevated in the tumor-draining lymph nodes. Similarly, recruitment of myeloid immune cells from the blood favors an immunosuppressive microenvironment in pre-metastatic lymph nodes, facilitating cancer cell growth and expansion, resulting in impaired recruitment of naïve lymphocytes and the antitumor immune response.[17,18]
Angiogenesis
Cancer cells that enter the lymphatics need to survive in a low-oxygen environment. Angiogenesis is induced in response to hypoxic environments. Cancer cells in the subcapsular sinus invade the lymph node, where they utilize the native vasculature of the lymph node. As the disease progresses, remodeling of endothelial vessels causes them to lose surface molecules and this alters their function.[19] Although there is angiogenesis, functional lymphatic vessels are restricted to the tumor margin and peritumor regions surrounding the tumors. As tumors lack functional lymphatic vessels within, the interstitial fluid pressure is elevated, which alters the lymph flow to the tumor-draining lymph nodes resulting in lymphedema.[20]
LYMPHEDEMA SECONDARY TO TREATMENT OF MALIGNANCY
In Western countries, secondary lymphedema is most often due to lymphatic injury sustained during the course of cancer treatment as seen following extensive lymph node dissection or adjuvant radiation therapy.
Surgery-induced lymphedema
Lymphadenectomy as part of treatment for malignancies especially when there is complete excision of a lymph node basin, directly disrupts the normal return of lymphatic fluid from the extremities.[21] In general, the risk of lymphedema is proportional to the number of lymph nodes sampled, with the excision of certain lymph nodes and lymph node basins posing a higher risk. Apart from injury, current evidence suggests that a variety of key players, including T helper cells, Tregs, macrophages, and dendritic cells, play complex roles in the pathology [Table 2] of the disease by releasing inflammatory cytokines and regulating the development of collateral lymphatic vessels.[22,23]
| Inflammatory cells | Role in lymphedema | |
|---|---|---|
| 1 | CD4 | Releases Th2 cytokines that cause progressive obliteration of the superficial and deep lymphatic systems with worsening lymphatic function and inadequate collateral lymphatic growth |
| 2 | T regs | Attenuate the severity of inflammatory tissue responses, local impaired adaptive immune response leads to recurrent soft tissue infections |
| 3 | Macrophages | Produce and activate transforming growth factor-β1, M2 macrophages are immunosuppressive, M2 macrophages cause regeneration of collateral lymphatics after lymphatic injury, significant source of IL-6 which regulates chronic inflammation and adipose metabolism and promote the expression of inducible nitric oxide synthase, which attenuates lymphatic vessel contraction in inflammation |
| 4 | Dendritic cells | Produce pro-inflammatory mediators that contribute to the ongoing cycle of inflammation |
Radiation-induced lymphedema
The immediate effect of direct radiation exposure on lymphatic vessels is minimal as demonstrated in both in vitro and in vivo studies, as structural and functional integrity are maintained. Damage to the lymphatic vessels occurs in a delayed fashion after radiation as the surrounding tissue turns into dense fibrous tissue that compresses and blocks the lymphatic flow.[24] Moreover, the proliferation of lymphatic vessels is inhibited by radiotherapy by prevention of compensatory lymphatic vessel growth. This further worsens the lymphedema.[25] Unlike lymphatic vessels which are insensitive to radiation, lymph nodes are highly radiosensitive.[26] In response to radiation, the initial change in lymph nodes is depletion of lymphocytes, followed by fatty change and eventually fibrosis. Fibrosis of lymph nodes significantly alters their ability to filter lymphatic fluid, increasing the pressure proximally which promotes lymphedema. Furthermore, there is a stronger propensity for the lymph node to transform into fibrous tissue after radiotherapy if the nodal basin is affected by regional metastasis.[27] Therefore, patients with lymph node metastasis undergoing radiotherapy are at an increased risk for lymphedema compared to irradiated patients without lymph node involvement.
LYMPHEDEMA SECONDARY TO CHRONIC VENOUS INSUFFICIENCY (CVI)
Fluorescence microlymphography undertaken in patients with severe CVI compared with that of healthy controls demonstrated obliteration of parts of the lymphatic superficial capillary network, cutaneous reflux of lymph from deep to superficial channels, and increased lymphatic capillary permeability.[28] Histological studies on skin biopsies taken from patients with CVI have demonstrated structural changes in dermal lymphatic vessels. There is collapse of the lumen of intradermal lymphatics, loss of the open intercellular junctions, and damage to the anchoring filaments which maintain the patency of the lymphatic vessel.[29] As mentioned above, these features lead to disruption of the normal unidirectional transport mechanism of the initial lymphatics and consequent impairment of lymphatic function. In severe CVI, lipodermatosclerosis may occur with ulceration.[30] A histological study in lipodermatosclerosis has shown a complete absence of lymphatics in the ulcer bed and a marked decrease in the number of lymphatics surrounding the ulcer. Additional findings observed included destruction of the endothelium and muscle lining of lymphatics draining the region.[30,31] Therefore, there are clinical and laboratory evidences to suggest that in CVI, in addition to the pathological changes in blood vessels, there is a concomitant pathology in lymphatics which leads to a deterioration in lymphatic function.
LYMPHEDEMA IN INFLAMMATORY SKIN DISEASES
Well-functioning, lymphatic pumping avoids swelling. The normal lymphatic activity ensures an adequate immune response since lymph transports antigens and immune cells. However, in inflammatory conditions, mediators, such as nitric oxide and prostaglandins that are abundantly produced, alter the lymphatic pumping. Most of them slow down the frequency of the contractions, in turn, slowing down the lymph flow and potentially triggering the swelling.[32] Further experimental examinations have revealed that other molecules, namely, cytokines such as TNF-α and IL-1 that are critical in the initial steps of an inflammatory reaction play an important role in mediating the lymphatic dysfunction.[23] Studies by Avraham and Zampell using animal models of lymphedema have implicated CD4+ T cells as the primary cells responsible for more than 70% of the inflammatory response.[33]
LYMPHEDEMA IN OBESITY
The etiological link between obesity and lymphedema is due to the increased production of lymph from an enlarging limb that compromises the capacity of the lymphatic system. External compression of lymphatics by adipose tissues, or even direct injury to the lymphatic endothelium by changes in body weight or diet can play a role in obesity-associated lymphedema. Obesity increases the risk of lymphedema in patients with lymphatic injury. Recent studies have shown that obese individuals can develop lymphedema even without antecedent surgery or injury. It has been proposed that body mass index, at the time of breast cancer diagnosis is a strong indicator for developing lymphedema than weight gain following treatment.[34] Histologic studies on clinical samples and animal models of lymphedema have shown that adipose deposition in the lymphedematous tissues can be compared to fat depots in obese patients. Similar to obesity, lymphedema-associated fat deposition results from both proliferation and hypertrophy of local adipocytes, and the resulting adipose depots are chronically inflamed and infiltrated by macrophages and lymphocytes.[34,35] In addition, lymphedematous tissues exhibit evidence of adipocyte death and phagocytosis by macrophages, producing an appearance of so-called “crown-like structures.” This is important because the production of inflammatory cytokines by these structures is associated with both an increased risk and aggressive behavior of a variety of malignancies in obese patients.[35]
LYMPHEDEMA SECONDARY TO IMMOBILITY
Movement and exercise help lymph drainage because muscle activity surrounding the lymphatic vessels massages fluid into and along them. Reduced mobility can, therefore, lead to lymphoedema because the fluid in the lymphatic system does not get moved along.[36]
In the systematic review conducted by Kwan et al., it was found that in the breast cancer patients, the benefits to be gained by exercise in the prevention of post-treatment lymphedema far outweigh the minimal adverse effects reported.[37] Therefore, the slowly progressive exercise of varying modalities is recommended to prevent the development or exacerbation of breast cancer-related lymphedema.[37] This again asserts the role of movement and exercise as opposed to immobility in the prevention of lymphedema.
LYMPHEDEMA SECONDARY TO PODOCONIOSIS
This geochemical, obliterative endolymphangitis, resulting in lymphatic obstruction and the clinical consequence of gross lower leg lymphedema, is common in alkaline volcanic tropical highlands. The development of podoconiosis is closely associated with barefoot walking on irritant soils. Farmers are at high risk, but the risk extends to any occupation that demands prolonged contact with the soil, and the condition has been noted among goldmine workers and weavers who sit at a ground level loom.[38] Regarding pathogenesis, a possible genetic predisposition has been suggested. DRB1*0701, DQA1*0201, and DQB1*0202 alleles are suspected to have a functional role in antigen presentation to T cells, that in turn, induces the immune response to soil antigen or mineral leading to development of the disease. Colloid-sized particles of elements common in irritant clays (aluminum, silicon, magnesium, and iron) are absorbed through the foot and have been demonstrated in macrophages in the lymph nodes of the lower limbs of those affected. Electron microscopy shows local macrophage phagosomes to contain particles of stacked kaolinite (Al2Si2O5(OH)4), while light microscopy shows subendothelial edema and subsequent collagenization of afferent lymphatics that cause narrowing and eventual obliteration of the lumen.[39]
LYMPHEDEMA IN PRETIBIAL MYXEDEMA
Autoantibodies directed against the thyroid-stimulating hormone receptor on thyroid follicular cells lead to hyperthyroidism of Graves’ disease. Pretibial myxoedema is a manifestation of Graves’ disease and occurs due to the deposition of glycosaminoglycans (mucin) within the dermis. Autoimmune, cellular, and mechanical factors play a role in the deposition of glycosaminoglycans in Grave’s disease. Lymphedema is an outcome of the obstruction of dermal lymphatic vessels by mucin.[2]
TRAUMA-INDUCED LYMPHEDEMA
The lymphatic system may be affected following closed traumatic upper limb or lower limb fractures or following surgery (e.g., shoulder arthroplasty).[40-42] Lymphoscintigraphy scans have shown an enlargement of lymphatics and lymph nodes that drain the site of injury or bone fracture (when the fracture heals without complications). Although the pathogenesis underlying the lymphatic response to a bone fracture is unclear, both clinical and experimental observations indicate that an inflammatory process triggered by invading bacteria or self-antigens exposed during trauma may lead to the persistent post-traumatic edema.[41]
FACTITIOUS LYMPHEDEMA
Factitious lymphedema can be caused by tourniquets, blows to the arm or repeated skin irritation. Usually, it is seen in patients with known psychiatric conditions.[43] Constriction using a tourniquet initially results in a flow disorder affecting venous return and this, in turn, causes rapid overwhelming of the lymphatic flow leading to dermal reflux. The degree of lymphatic compromise will depend on the force and duration of pressure exerted by the tourniquet. Prolonged pressure can lead to complications typical of chronic lymphstasis.[44]
TRUNCAL LYMPHEDEMA
Truncal lymphedema frequently develops following the treatment of breast or lung cancer and can be present with or without significant involvement of the adjacent arm. Disruption of the lymphatic drainage pathways can occur if there is a complete or partial removal of the axillary lymph nodes. This causes swelling of the breast wall and the chest area. Scars following breast surgery such as lumpectomy, mastectomy, or reconstructive breast surgery can further disrupt the natural lymphatic drainage pattern. Radiation treatments can form fibrotic tissues in the chest wall or armpit and cause truncal lymphedema.[45]
NEWER CONCEPTS
A complex pathological interplay is suspected to be the underlying mechanism of lymphatic dysfunction [Figure 1] and new research explores new theories.[46] Stimulation of collagen production by fibroblasts, disruption of elastic fibers, activation of keratinocytes, and adipocytes tissue expansion are proposed to aggravate the development of lymphedema.
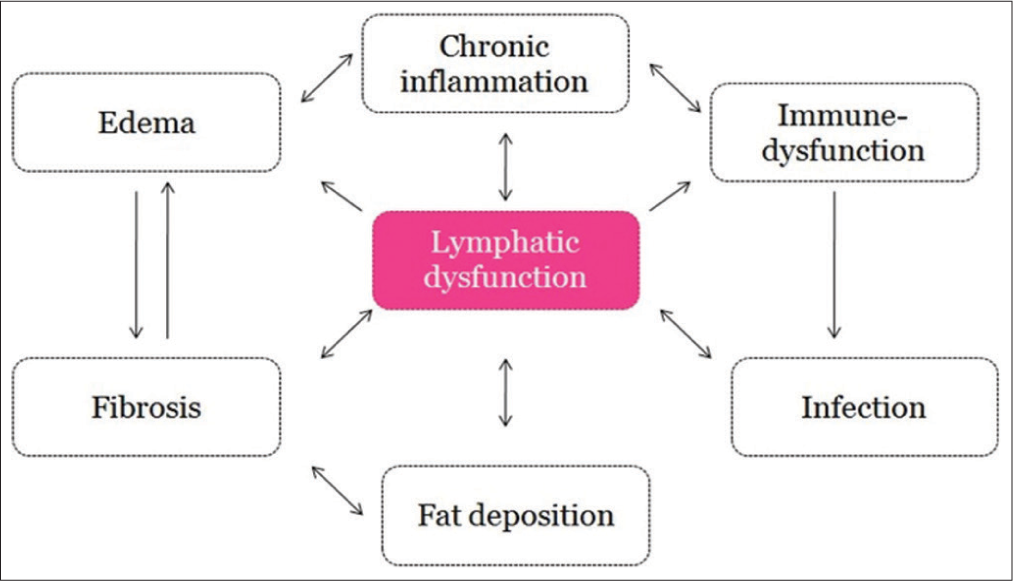
- Complex pathomechanisms involved in secondary lymphedema.
ADIPOSE TISSUE EXPANSION AND REMODELING IN LYMPHEDEMA
Current evidence demonstrates that tissue swelling in lymphedema is due to fat deposition and not just due to the accumulation of fluid. Adipose tissue hypertrophy in lymphedema is accompanied by adipose remodeling, similar to what occurs in obesity.[47] Observations show that hypertrophic fat lobules compress and collapse their feeding lymphatic capillaries, resulting in a vicious cycle of disruption of fluid and lipid transport, ultimately leading to further fat accumulation in the periphery.[35,48]
FIBROSIS IN LYMPHEDEMA
Mihara et al. had demonstrated that histological and immunohistochemical examination of skin tissues from clinical and experimental lymphedema showed increased amounts of collagen fibers in the edematous skin.[49] Fibrosis in lymphedema is not confined to the dermis, but may extend to the subcutaneous tissue including the adipose tissue. Hypertrophic adipocytes in human lymphedema patients exhibit thick fibrous matrix between lobules. This hardens lymphedematous tissues, resulting in non-pitting edema.[50] Collecting lymphatic vessels play a role in lymphedema depending on the manner of collagen deposition. Normal type of collecting lymphatic vessels have collagen fibers and smooth muscle cells within the medial layer. Ectasis type lymphatics are characterized by the dilation of the lymphatic vessel walls, with long and elongated collagen fibers. Contraction type lymphatics show the deposition of thick collagen fibers mixed with smooth muscle cells in the medial layer. The thick collagen fibers impair vessel contraction, resulting in loss of function in the collecting lymphatic vessels. Sclerosis-type vessels exhibit an increase in smooth muscle cells and collagen fibers and a reduction in their ability to transport lymph fluid, causing excessive lymph leakage. These changes in collecting vessels are consistent with the previous findings that show decreased lymph vessel contractility in acquired lymphedema. In addition, fibrosis in the skin and subcutaneous tissue may worsen lymphatic dysfunction by directly inhibiting lymphatic endothelial cell proliferation leading to inhibition of sprouting and branching of new lymphatics.[46,51]
CONCLUSION
To summarize, the multidimensional pathophysiological mechanism of lymphedema includes processes such as lymph stasis, lymphatic vessel remodeling, lymphatic dysfunction, inflammation, adipose tissue deposition, and fibrosis. However, it is often not possible to sequentially arrange these events due to the complex interactions between the pathomechanisms. Moreover, studies have shown that certain events predominate depending on the triggering factors such as infection, malignancy, inflammation, surgery, radiotherapy, and concomitant venous stasis and so on. More studies focused on the etiology of lymphedema are needed to delineate the types of tissue changes across the stages and causes of lymphedema. A better understanding of the pathophysiology of lymphedema and its cellular and molecular mediators will pave way for novel therapeutic approaches for this chronic and debilitating condition.
Declaration of patient consent
Not required as there are no patients in this article.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Anatomy of the lymphatic system and the lymphosome concept with reference to lymphedema. Sem Plast Surg. 2018;32:5-11.
- [CrossRef] [PubMed] [Google Scholar]
- Disorders of the lymphatic vessels In: Chalmers R, Barker J, Griffiths C, Bleiker T, Creamer D, eds. Rook's Textbook of Dermatology Vol 105. (9th ed). United Kingdom: John Wiley and Sons Limited; 2016. p. :1-63.
- [Google Scholar]
- Insights into the pathogenesis of disease in human lymphatic filariasis. Lymphat Res Biol. 2013;11:144-8.
- [CrossRef] [PubMed] [Google Scholar]
- A role for tumour necrosis factor-alpha in acute lymphatic filariasis. Parasite Immunol. 1996;18:421-4.
- [CrossRef] [PubMed] [Google Scholar]
- Human bancroftian filariasis: Immunological markers of morbidity and infection. Microbes Infect. 2006;8:2414-23.
- [CrossRef] [PubMed] [Google Scholar]
- Serum levels of endothelin-1 (ET-1), interleukin-2 (IL-2) and amino-terminal propeptide type III procollagen (PIII NP) in patients with acute and chronic filariasis. J Egypt Soc Parasitol. 2001;31:169-76.
- [Google Scholar]
- Lymphatics in human lymphatic filariasis: In vitro models of parasite-induced lymphatic remodeling. Lymphat Res Biol. 2009;7:215-219.
- [CrossRef] [PubMed] [Google Scholar]
- Lymphangiogenesis: Mechanisms, significance and clinical implications. EXS. 1997;79:65-112.
- [CrossRef] [PubMed] [Google Scholar]
- Doxycycline reduces plasma VEGF-C/sVEGFR-3 and improves pathology in lymphatic filariasis. PLoS Pathog. 2006;2:e92.
- [CrossRef] [PubMed] [Google Scholar]
- Challenges of cellulitis in a lymphedematous extremity: A case report. Cases J. 2009;2:9377.
- [CrossRef] [PubMed] [Google Scholar]
- Chronic peripheral oedema: The critical role of the lymphatic system. Clin Med. 2004;4:448-53.
- [CrossRef] [PubMed] [Google Scholar]
- Scrofuloderma leading to lymphedema of external genitalia and lower extremities. Plast Reconstr Surg. 1977;59:436-8.
- [CrossRef] [PubMed] [Google Scholar]
- Pseudoelephantiasis of vulva of tuberculous etiology. Indian J Dermatol Venereol Leprol. 1968;34:245-7.
- [Google Scholar]
- Final trial report of sentinel-node biopsy versus nodal observation in melanoma. N Engl J Med. 2014;370:599-609.
- [CrossRef] [PubMed] [Google Scholar]
- A new concept toward the prevention of lymphedema: Axillary reverse mapping. J Surg Oncol. 2008;97:563-4.
- [CrossRef] [PubMed] [Google Scholar]
- Identifying gaps in the locoregional management of early breast cancer: Highlights from the Kyoto consensus conference. Ann Surg Oncol. 2011;18:2885-92.
- [CrossRef] [PubMed] [Google Scholar]
- Living with lymphedema: A qualitative study of women's perspectives on prevention and management following breast cancer-related treatment. Can Oncol Nurs J. 2006;16:165-79.
- [CrossRef] [PubMed] [Google Scholar]
- A systematic review of care delivery models and economic analyses in lymphedema: Health policy impact (2004-2011) Lymphology. 2013;46:27-41.
- [Google Scholar]
- Lymphedema-related angiogenic tumors and other malignancies. Clin Dermatol. 2014;32:616-20.
- [CrossRef] [PubMed] [Google Scholar]
- Upper-body morbidity following breast cancer treatment is common, may persist longer-term and adversely influences quality of life. Health Qual Life Outcomes. 2010;8:92.
- [CrossRef] [PubMed] [Google Scholar]
- Lower extremity lymphedema in patients with gynecologic malignancies. Int J Gynecol Cancer. 2020;30:252-60.
- [CrossRef] [PubMed] [Google Scholar]
- CD4(+) cells regulate fibrosis and lymphangiogenesis in response to lymphatic fluid stasis. PLoS One. 2012;7:e49940.
- [CrossRef] [PubMed] [Google Scholar]
- Inflammatory manifestations of lymphedema. Int J Mol Sci. 2017;18:171.
- [CrossRef] [PubMed] [Google Scholar]
- The impact of radiation on lymphedema: A review of the literature. Gland Surg. 2020;9:596-602.
- [CrossRef] [PubMed] [Google Scholar]
- Lymphedema in gynecologic cancer survivors: An area for exploration? Cancer Nurs. 2007;30:E11-8.
- [CrossRef] [PubMed] [Google Scholar]
- The effect of radiation on the lymph and on the lymph vessels. Radiology. 1963;80:814-7.
- [CrossRef] [PubMed] [Google Scholar]
- The effects of irradiation (external and internal) on lymphatic dynamics. Am J Roentgenol Radium Ther Nucl Med. 1967;99:404-14.
- [CrossRef] [PubMed] [Google Scholar]
- Fluorescence microlymphography in chronic venous incompetence. Int Angiol. 1989;4(Suppl):23-6.
- [Google Scholar]
- Morphological changes of dermal blood and lymphatic vessels in chronic venous insufficiency of the leg. Int Angiol. 1994;13:308-11.
- [Google Scholar]
- Morphology of lymphatics in human venous crural ulcers with lipodermatosclerosis. Lymphology. 2001;34:11-123.
- [Google Scholar]
- Lymphatic microangiopathy: A complication of severe chronic venous incompetence. Lymphology. 1982;15:60-5.
- [Google Scholar]
- Lymphatic pumping: Mechanics, mechanisms and malfunction. J Physiol. 2016;594:5749-68.
- [CrossRef] [PubMed] [Google Scholar]
- Th2 differentiation is necessary for soft tissue fibrosis and lymphatic dysfunction resulting from lymphedema. FASEB J. 2013;27:1114-26.
- [CrossRef] [PubMed] [Google Scholar]
- Lymphedema and obesity: Is there a link? Plast Reconstr Surg. 2014;134:154e-60.
- [CrossRef] [PubMed] [Google Scholar]
- Regulation of adipogenesis by lymphatic fluid stasis: Part I. Adipogenesis, fibrosis, and inflammation. Plast Reconstr Surg. 2012;129:825-34.
- [CrossRef] [PubMed] [Google Scholar]
- A safe and effective upper extremity resistive exercise program for woman post breast cancer treatment. Rehabil Oncol. 2008;26:3-10.
- [CrossRef] [Google Scholar]
- Exercise in patients with lymphedema: A systematic review of the contemporary literature. J Cancer Surviv. 2011;5:320-36.
- [CrossRef] [PubMed] [Google Scholar]
- Podoconiosis: Endemic nonfilarial elephantiasis. Curr Opin Infect Dis. 2005;18:119-22.
- [CrossRef] [PubMed] [Google Scholar]
- HLA class II locus and susceptibility to podoconiosis. N Engl J Med. 2012;366:1200-8.
- [CrossRef] [PubMed] [Google Scholar]
- Lymphedema of the hand and forearm following fracture of the distal radius. Orthopedics. 2008;31:172.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of lymphedema on the recovery of fractures. J Orthop Sci. 2007;12:578-84.
- [CrossRef] [PubMed] [Google Scholar]
- Shoulder arthroplasty in patients with upper extremity lymphedema may result in transient or permanent lymphedema worsening. Shoulder Elbow. 2020;12(Suppl 1):53-60.
- [CrossRef] [PubMed] [Google Scholar]
- The upper extremity and psychiatric illness. J Hand Surg Am. 1985;10:687-93.
- [CrossRef] [Google Scholar]
- Lymphatic outflow scintigraphy in a case of artificial oedema of the lower limb. Nuklearmedizin. 1994;33:268-70.
- [CrossRef] [PubMed] [Google Scholar]
- Breast cancer-related lymphedema: Symptoms, diagnosis, risk reduction, and management. World J Clin Oncol. 2014;5:241-7.
- [CrossRef] [PubMed] [Google Scholar]
- The unresolved pathophysiology of lymphedema. Front Physiol. 2020;11:137.
- [CrossRef] [PubMed] [Google Scholar]
- Serum immune proteins in limb lymphedema reflecting tissue processes caused by lymph stasis and chronic dermato-lymphangio-adenitis (Cellulitis) Lymphat Res Biol. 2017;15:246-51.
- [CrossRef] [PubMed] [Google Scholar]
- Pathological changes of adipose tissue in secondary lymphoedema. Br J Dermatol. 2017;177:158-167.
- [CrossRef] [PubMed] [Google Scholar]
- Pathological steps of cancer-related lymphedema: Histological changes in the collecting lymphatic vessels after lymphadenectomy. PLoS One. 2012;7:e41126.
- [CrossRef] [PubMed] [Google Scholar]
- Application of multiphoton imaging and machine learning to lymphedema tissue analysis. Biomed Opt Express. 2019;10:3353-68.
- [CrossRef] [PubMed] [Google Scholar]
- Fibrosis worsens chronic lymphedema in rodent tissues. Am J Physiol Heart Circ Physiol. 2015;308:H1229-36.
- [CrossRef] [PubMed] [Google Scholar]






