Translate this page into:
Biofilm in dermatology
*Corresponding author: Dr. Keerankulangara Devi, Department of Dermatology, Government Medical College, Kozhikode, Kerala, India. drdevi64@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Vaishnavi KV, Safar L, Devi K. Biofilm in dermatology. J Skin Sex Transm Dis 2019;1:3-7.
Abstract
Biofilms represent densely packed aggregates of microorganisms encased in a self-produced matrix of extracellular polymeric substance, helping in their attachment to biotic and abiotic surfaces conferring them survival advantage in unfavorable conditions. The stages in biofilm formation are complex, the knowledge of which is important as their role in a diverse range of dermatological diseases is being constantly unraveled. Due to their chronic persistent nature, inability of routine culture techniques to detect them and their resistance to standard antimicrobial therapy, they pose a unique challenge to the treating clinician. Although various novel treatment options are available, they show varying degrees of efficacy and the eradication of biofilm in cutaneous diseases still remains enigmatic. Hence, better understanding of their molecular biology, pathogenesis, and role in various diseases can help in the development of potential therapeutic strategies against biofilms in the future.
Keywords
Biofilm
Dermatology
Antimicrobial therapy
INTRODUCTION
Biofilm was first described in the 17th century, when Anton Von Leeuwenhoek, the inventor of the microscope, saw aggregates of microbes on scrapings of plaque from his teeth. The term “Biofilm” was coined by Bill Costerton, in 1978.
Microorganisms exist on biotic and abiotic surfaces as individual free-floating planktonic forms or as multicellular consortiums known as biofilms. Within a biofilm, the organisms are embedded in a glycocalyx. The glycocalyx is a self-produced matrix of extracellular polymeric substance (EPS) which consists of polysaccharides, lipids, proteins, and extracellular DNA.[1] The EPS is considered to be the hallmark of biofilm formation, which helps in attachment of microbiological communities to the surfaces.[1] The transition from planktonic form to biofilm is regulated by multiple factors including bacterial cell density, nutrient availability, and use of antimicrobials.[1]
Biofilms have been demonstrated on various biological surfaces such as teeth, heart valves, ear mucosa, prosthetic valves, dental and orthopedic implants, contact lenses, and intravenous catheters.[1]
Steps involved in biofilm formation are shown in Figure 1.
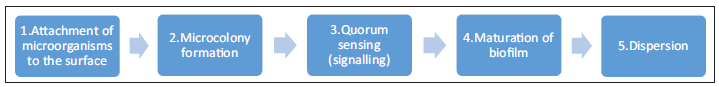
- Figure 1: Steps in biofilm formation.
The formation of a biofilm begins with the attachment of free-floating microorganisms to a surface followed by microcolony formation. During surface colonization, the organisms are able to communicate using quorum sensing (QS) products such as N-acyl homoserine lactone. A polysaccharide matrix encloses bacterial biofilms and they mature. This is followed by the final stage of dispersion.[2]
Biofilms offer unique advantages to the organisms including protection from host defenses, metabolic cooperation, increased virulence, differential gene expression, and increased resistance to antimicrobials.[2] Several in vitro studies show that the bacteria in biofilms are 50–500 times more resistant to antibiotics than their planktonic forms.[3-5] Several factors including physical barrier, altered growth and metabolism, increased mutation, gene transfer, phenotype switching, persisters or spore-like forms, increased efflux pumps, and production of enzymes contribute to antimicrobial tolerance.[4]
Biofilms cannot be easily visualized in skin biopsies with routine light microscopy and require special techniques such as electron microscopy, epifluorescence microscopy, peptide nucleic acid-fluorescence in situ hybridization, cryo-scanning, electron microscopy, or confocal laser scanning microscopy.[4] Biofilms are associated with various pathological conditions in humans such as cystic fibrosis, colonization of indwelling medical devices, and dental plaque formation involved in caries and periodontitis.
Epithelial biofilms have been implicated in number of dermatological conditions including chronic wounds, atopic dermatitis, hidradenitis suppurativa, candidiasis, acne vulgaris, and onychomycosis.
Since biofilms pose a challenge to the clinician due to the limitation of routine diagnostic culture techniques for their detection and their resistance to conventional antimicrobial therapy due to their persistent and chronic nature, hence awareness of the concept of biofilm is necessary [Figure 2].

- Figure 2: Dermatological conditions in which biofilms are implicated.
CHRONIC WOUNDS
Open wounds have increased chance of biofilm formation due to the lack of cutaneous protection.[3] Biofilms can develop on wounds very rapidly in as little as 8 h.[4] Biofilms of Staphylococcus aureus were found to cause increased resistance to antibiotic therapy compared to its planktonic state and also found to delay wound healing by delaying reepithelialization and hindering the development of granulation tissue.[5] Biofilms may be found in 60% of chronic wounds and 6% of acute wounds.[5] James et al. identified Pseudomonas aeruginosa, S. aureus, and Enterococcus as main culprits in biofilm formation on wounds.[5,6] It was also found that S. aureus has the capacity to form thickest biofilm in medium composed of plasma and glucose and heaviest biofilm load was found in diabetic wounds.[5]
The prevention and treatment of biofilm can be achieved with repeated wound debridement or desloughing to remove the non-viable tissue, thus reducing the surface area for biofilm to form upon. Low-frequency ultrasound, lasers, and photodynamic therapy (PDT) are alternative non-invasive methods to achieve biofilm breakdown and enhance wound healing. Various studies have shown that the addition of lactoferrin and xylitol to hydrogel dressing reduced biofilm formation in chronic wounds. Kravvas et al. studied the enhanced antibiofilm efficacy on adding silver nitrate in wound dressing that reduced the biofilm viability. Application of dressing containing polyhexanide resulted in complete healing of 12 of 16 chronic wounds.[3,7,8]
The recent adjunctive antibiofilm treatment is application of platelet and platelet growth factor. Rozalsk et al. studied the efficacy of platelet-rich plasma in wounds and found that it reduced the population of S. aureus in their planktonic cultures by 56–87% and decreased biofilm formation by 7–38%. Future antibiofilm therapies may target QS, where RNA III-inhibiting peptide, a specific staphylococcus QS inhibitor, can cause deficient biofilm and better healing.[9]
HIDRADENITIS SUPPURATIVA
Several studies hypothesize the synergistic interaction between commensal microbials and aberrant innate immunity as the major factors contributing to the pathogenesis of hidradenitis suppurativa.[10-12] A study conducted by Jahns et al. found that 7 of 27 patients with hidradenitis suppurativa had the presence of bacterial biofilms in hair follicle and sinus tracts.[13] Ring et al. found that biofilm found in 67% of chronic lesions was localized in sinus tracts and infundibulum.[10] It was postulated that localization of biofilm in sinus tracts and infundibulum was due to abundant keratinous debris that may provide nidus to commensal cocci and anaerobes in these lesions. Hence, it was suggested that to attain better disease control, combining medical therapy with early surgical excision or simple deroofing can be attempted.[13]
ATOPIC DERMATITIS
Biofilm has a major role in the pathogenesis of atopic dermatitis.[14,15] It is known that S. aureus can be identified more frequently in atopic dermatitis lesions and atopic skin than from normal skin and is implicated in its pathogenesis.[16,17] A study conducted by Katsuyama et al. revealed that the biofilm-related antibiotic resistance of S. aureus along with its rapid growth contributes to its colonization in atopic skin.[16] It was proposed that the biofilm-induced occlusion of sweat ducts could be the cause of inflammation and pruritus in atopic dermatitis.[14,18] Ikezawa et al. demonstrated an effective topical treatment of S. aureus biofilm using farnesol and xylitol that inhibited different stages of biofilm formation independently.[18,19] Further studies showed the efficacy of emollients in preventing biofilm formation. It was found out that treatment of atopic dermatitis lesions by lipid replenishing balm inhibited the adhesion of S. aureus to the skin and thus prevented biofilm formation.[20]
CANDIDIASIS
Candida albicans is one among those fungi that are found as commensals on mucocutaneous surfaces which may cause opportunistic infections in humans when there is any alteration in the host immunity or local ecology.[21,22] Studies demonstrate the ability of Candida in forming biofilms on mucosal surfaces, suggesting its role in the pathogenesis of these infections.[22] It is presumed that oral thrush with its characteristic white plaque is due to biofilm production.[21,22] Role of Candida biofilm in intertrigo has not been studied yet. Factors responsible for the protection of C. albicans from neutrophilic attack and immune mechanism were studied and it was found that BCR-1 transcription factor was involved in regulating biofilm formation and virulence.[22] Denture stomatitis or chronic atrophic candidiasis due to ill-fitting denture causes altered mucosal barrier, leading to Candida biofilm formation.[23] Candidal biofilm has 30–2000-fold increased resistance to amphotericin B, fluconazole, itraconazole, and ketoconazole as compared to its planktonic form.[23] Newly detected drugs effective against C. albicans biofilm are echinocandins and liposomal amphotericin B that is only available as parenteral preparations at present. QS inhibitors, vaccines, anticandidal antibodies, cytokines therapy, and specific BCR1 inhibitors are other experimental therapeutic options against Candida biofilms.[24]
ACNE
Studies indicate that Propionibacterium acnes can form biofilms in vitro and on implants. This was first clearly hypothesized by Burkhart and Burkhart, in 2003.[25] This is aided by autoinducer-2 which acts as a QS molecule. Within this biofilm, bacteria become organized to make the use of available nutrients and exhibit microheterogeneity being controlled by various genes. The biofilm of P. acnes helps in follicular plugging and cohesiveness which is key to the pathogenesis of acne vulgaris.[26] Various enzymes such as lipases, hyaluronidases, and chemotactic factors are secreted in P. acne biofilm increasing the free fatty acid concentration available as nutrient source for the bacterium and further perpetuating inflammation. In addition, TLR2 and TLR4 are activated to bind to biofilms which activate NFkB, leading to robust proinflammatory response in acne. Well nested in the biofilms, P. acnes are protected from the effect of antimicrobials necessitating long-term treatment for acne with sometimes little success. Isotretinoin which works by decreasing sebaceous gland size and sebum depletes P. acnes of the available nutrients and thus the microenvironment for the existence of its biofilm. Coenye et al. have found the efficacy of certain plant extracts such as icariin and resveratrol to exhibit antibiofilm activity.[27] Agents such as silver, selenium, curcumin, flavonoids, and rifampicin were also found to have biofilm-dispersing property. With respect to the antibiotics, highly fat-soluble minocycline achieves high therapeutic concentration explained by the biofilm concept. The use of recombinant human DNase and other drugs which produce hydroxyl radical altering the biofilm microenvironment can also show efficacy against biofilms in acne.[25]
ONYCHOMYCOSIS
Fungal biofilms form in the nail unit and contribute to the chronic nature of onychomycosis. The strongest evidence for the biofilm formation is for Candida and non-dermatophyte species.[28] Burkhart and Burkhart have documented that the fungal cells found in recalcitrant dermatophytomas are avidly attached to the nail plate conferring advantages for growth, survival, and chronicity and accounting for difficulty in treatment. The biofilm concept explains the boosted oral antifungal treatment for dermatophytomas. Since minimum inhibitory concentration has been calculated based on antifungal susceptibility of planktonic forms, they may not be as translatable to the clinical scenario. Various agents such as liposomal amphotericin B, echinocandins, mucolytics, miltefosine, and propolis resin from bees have shown antibiofilm properties in onychomycosis. The use of enzymes such as DNase, alpha-amylase, chitosan, povidone-iodine, and physical modalities such as lasers (NdYaG, IPL, and near-infrared light) and low-frequency surface acoustic waves with PDT has all been investigated for their antibiofilm activity with varying success.[29] Other agents such as antibody-mediated inhibition of matrix polysaccharides, lactoferrin, antimicrobial peptides (AMP), and QS molecules are under trial.[29]
DERMAL FILLERS
The adverse reactions to dermal fillers, especially the long-acting ones in the form of erythematous tender nodules, abscesses, and sinuses, may occur weeks to months after their administration. They have been attributed to allergic or foreign body reactions for a long time. They are now considered to be of infectious etiology, are often culture negative, and are attributed to biofilms. Studies indicate that the presence of biofilms of P. acnes, Staphylococcus, and Pseudomonas was isolated from biopsy samples of filler granulomas. This is likely to represent bacterial contamination during filler injection therapy which points to the role of broad- spectrum antibiotics for the treatment and resolution of the lesions. Steroids should be avoided and may even worsen the condition. Incision and drainage for fluctuant lesions and a biopsy with culture are recommended for late-onset reactions keeping in mind the fact that cultures often yield no organism. Finally, proper standard of care should be adopted to prevent such occurrence by thorough antiseptic cleansing of area before the injection and preventive antibiotic prophylaxis.[30] Wang et al. have found that the bacterial transfer is increased with the use of increased needle diameter and decreased injection depth and the use of fanning technique of injection.[31] The use of hyaluronidase, lasers, and surgical excision may be advocated in special situations. Recently, the use of AMP has been shown to disrupt biofilms when used incorporated to antiseptics or onto filler needles. AMP bind to lipopolysaccharide on the cell wall of organisms forming biofilm through electrostatic interaction, altering the membrane potential, and inducing membrane permeability and thus destroying the biofilm.
OTHER CONDITIONS
Biofilms are implicated in the pathogenesis of miliaria where they cause occlusion of sweat glands.[24] Akiyama et al. identified the presence of Streptococcus pyogenes and S. aureus biofilms in impetiginized skin.[17] Similarly, Staphylococcus biofilms have been detected in specimens of pemphigus foliaceus.[17] Skin biofilms have also been implicated in the pathogenesis of rosacea.
Biofilms are hence found to be implicated in a wide array of dermatological conditions as described. Biofilms are usually resistant to the conventional antiseptics and antibiotics. The practical therapeutic approach varies depending on the underlying condition in which it is implicated. Therefore, the treatment may be aimed to disrupt biofilms by breaking down their extracellular matrix including wound debridement, early surgical excision, drugs interrupting biofilm microenvironment, and various physical modalities including pulsed ultrasound and lasers so that the antimicrobials can better access and kill the microbes.
CONCLUSION
Cutaneous microbiological flora is far more complicated than previously appreciated. Modern molecular technologies have unveiled the ubiquitous nature of biofilms and their role in pathogenesis of various dermatological disorders. Better understanding of biofilm characteristics will help in explaining chronic nature of many dermatological conditions and also revolutionize the therapeutic approach toward various diseases.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Microscopic and physiologic evidence for biofilm-associated wound colonization in vivo. Wound Repair Regen. 2008;16:23-9.
- [CrossRef] [PubMed] [Google Scholar]
- Biofilms and wounds: An overview of the evidence. Adv Wound Care (New Rochelle). 2015;4:373-81.
- [CrossRef] [PubMed] [Google Scholar]
- The increasing relevance of biofilms in common dermatological conditions. J Dermatolog Treat. 2018;29:202-7.
- [CrossRef] [PubMed] [Google Scholar]
- Staphylococcus aureus infection on cut wounds in the mouse skin: Experimental staphylococcal botryomycosis. J Dermatol Sci. 1996;11:234-8.
- [CrossRef] [Google Scholar]
- Alcoholic ingredients in skin disinfectants increase biofilm expression of Staphylococcus epidermidis. J Antimicrob Chemother. 2002;49:683-7.
- [CrossRef] [PubMed] [Google Scholar]
- Microsensor and transcriptomic signatures of oxygen depletion in biofilms associated with chronic wounds. Wound Repair Regen. 2016;24:373-83.
- [CrossRef] [PubMed] [Google Scholar]
- Slough and biofilm: Removal of barriers to wound healing by desloughing. J Wound Care. 2015;24:498-503.
- [CrossRef] [Google Scholar]
- Staphylococcal biofilms impair wound healing by delaying reepithelialization in a murine cutaneous wound model. Wound Repair Regen. 2009;17:354-9.
- [CrossRef] [PubMed] [Google Scholar]
- Normal skin microbiota is altered in pre-clinical hidradenitis suppurativa. Acta Derm Venereol. 2017;97:208-13.
- [CrossRef] [PubMed] [Google Scholar]
- Intrinsic defect in keratinocyte function leads to inflammation in hidradenitis suppurativa. J Invest Dermatol. 2016;136:1768-80.
- [CrossRef] [PubMed] [Google Scholar]
- Increased expression of integrin á6â4 in the basement membrane zone lining the sebaceous glands in hidradenitis suppurativa. Acta Derm Venereol. 2015;95:994-6.
- [CrossRef] [PubMed] [Google Scholar]
- Microbiology of hidradenitis suppurativa (acne inversa): A histological study of 27 patients. APMIS. 2014;122:804-9.
- [CrossRef] [PubMed] [Google Scholar]
- The presence and impact of biofilm-producing staphylococci in atopic dermatitis. JAMA Dermatol. 2014;150:260-5.
- [CrossRef] [PubMed] [Google Scholar]
- Management of atopic dermatitis in the pediatric population. Pediatrics. 2008;122:812-24.
- [CrossRef] [PubMed] [Google Scholar]
- A novel method to control the balance of skin microflora. Part 1. Attack on biofilm of Staphylococcus aureus without antibiotics. J Dermatol Sci. 2005;38:197-205.
- [CrossRef] [Google Scholar]
- Confocal laser scanning microscopic observation of glycocalyx production by Staphylococcus aureus in skin lesions of bullous impetigo, atopic dermatitis and pemphigus foliaceus. Br J Dermatol. 2003;148:526-32.
- [CrossRef] [PubMed] [Google Scholar]
- A role of Staphyococcus aureus interleukin-18, nerve growth factor and semaphorin 3A, an axon guidance molecule, in pathogenesis and treatment of atopic dermatitis. Allergy Asthma Immunol Res. 2010;2:235-46.
- [CrossRef] [PubMed] [Google Scholar]
- A novel method to control the balance of skin microflora part 2. A study to assess the effect of a cream containing farnesol and xylitol on atopic dry skin. J Dermatol Sci. 2005;38:207-13.
- [CrossRef] [Google Scholar]
- Atopic dermatitis In: Goldsmith L, ed. Fitzpatrick’s Dermatology in General Medicine (7). New York: McGraw Hill; 2008. p. :175-7. In: ed. p.
- [Google Scholar]
- Biofilm lifestyle of Candida A mini review. Oral Dis. 2008;14:582-90.
- [CrossRef] [PubMed] [Google Scholar]
- Candida albicans forms biofilms on the vaginal mucosa. Microbiology. 2010;156:3635-44.
- [CrossRef] [PubMed] [Google Scholar]
- Antifungal susceptibility of Candida biofilms: Unique efficacy of amphotericin B lipid formulations and echinocandins. Antimicrob Agents Chemother. 2002;46:1773-80.
- [CrossRef] [PubMed] [Google Scholar]
- The role of extracellular polysaccharide substance produced by Staphylococcus epidermidis in miliaria. J Am Acad Dermatol. 1995;33:729-33.
- [CrossRef] [Google Scholar]
- Expanding the microcomedone theory and acne therapeutics: Propionibacterium acnes biofilm produces biological glue that holds corneocytes together to form plug. J Am Acad Dermatol. 2007;57:722-4.
- [CrossRef] [PubMed] [Google Scholar]
- An increased incidence of Propionibacterium acnes biofilms in acne vulgaris: A case-control study. Br J Dermatol. 2012;167:50-8.
- [CrossRef] [PubMed] [Google Scholar]
- Eradication of Propionibacterium acnes biofilms by plant extracts and putative identification of icariin, resveratrol and salidroside as active compounds. Phytomedicine. 2012;19:409-12.
- [CrossRef] [PubMed] [Google Scholar]
- Evidence for biofilms in onychomycosis. G Ital Dermatol Venereol. 2019;154:50-5.
- [CrossRef] [Google Scholar]
- Antibiofilm treatment for onychomycosis and chronic fungal infections. Skin Appendage Disord. 2018;4:136-40.
- [CrossRef] [PubMed] [Google Scholar]
- Biofilms: Their role in dermal fillers. J Cutan Aesthet Surg. 2010;3:20-2.
- [CrossRef] [PubMed] [Google Scholar]
- Injections through skin colonized with Staphylococcus aureus biofilm introduce contamination despite standard antimicrobial preparation procedures. Sci Rep. 2017;7:450-70.
- [CrossRef] [Google Scholar]






