Translate this page into:
Drug-induced cutaneous small vessel vasculitis following vortioxetine
*Corresponding author: Lakshmi Chembolli, Former Professor of Dermatology, PSGIMSR, Peelamedu, Coimbatore, Tamil Nadu, India, Presently Dermatologist, Burjeel Medical Centre, Abu Dhabi, UAE. lakshmi.chembolli@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Chembolli L. Drug-induced cutaneous small vessel vasculitis following vortioxetine. J Skin Sex Transm Dis 2022;4:139-42.
Dear Editor,
Vortioxetine is a newer drug used in the treatment of major depressive disorder (MDD) since 2013.[1] It is a selective serotonin reuptake inhibitor (SSRI), but it also acts as a serotonin (5-hydroxy tryptamine, 5-HT) agonist, antagonist, and transport inhibitor.[2]
Vortioxetine increases serotonin levels in the central nervous system by inhibiting its reuptake. It is an antidepressant with a multimodal mechanism of action. It has 5-HT carrier inhibitor, 5-HT3, 5-HT7, and 5-HT1D receptor antagonist, 5-HT1B partial agonist, and 5-HT1A agonist properties.[2] Common side effects include nausea, headache, dry mouth, dizziness, diarrhea, and generalized pruritus.[1,3] Serious side effects include abnormal bleeding, hyponatremia, and activation of mania/hypomania which necessitate its discontinuation.[1] There is a risk of serotonin syndrome when vortioxetine is combined with other serotonergic drugs.
This report describes a patient who developed cutaneous small vessel vasculitis following vortioxetine.
A 42-year-old woman presented with intensely pruritic, crusted papules, distributed over the arms, scalp, abdomen, and jaw line for 10 months. The initial lesion was an isolated pruritic papule that appeared on the abdomen about 10 months back followed by crops of similar lesions with recent aggravation of symptoms in the past 6 months [Figure 1a]. A punched-out ulcer could be visualized on lifting the crust of the lesion on the jaw line [Figure 1b].

- (a) A papule with superficial crusting (solid black arrow) and an eroded papule (black arrow) on the upper arm of a patient receiving vortioxetine; (b) lesion on the jaw of the same patient revealing a punched-out ulcer on removal of the crust.
She had excoriated most of the lesions (since they were intensely pruritic) and could contribute to their crusted appearance.
The patient was on vortioxetine 10 mg daily for major depression since 1½ years. She had no other chronic illness and was not on any other medication. After a detailed evaluation, the possibility of an allergic reaction to vortioxetine was considered. She was advised a punch biopsy, complete hemogram, urine microscopy, and renal and liver function tests.
After discussing with her psychiatrist, we advised her to stop vortioxetine. She was treated with topical mometasone furoate 0.1% ointment twice daily and oral cetirizine 10 mg at night for 7 days. The lesions showed healing with the medication, but as the patient was hesitant to stop vortioxetine for fear of aggravating her psychiatric illness, she continued to take it at half the dose. She continued developing new lesions, but fewer in number.
Biopsy of a crusted papule from her arm revealed hyperkeratosis, acanthosis, and focal parakeratosis. The epidermis showed focal erosions with dense neutrophilic clusters within the stratum corneum. Neutrophils were present in the dermal inflammatory infiltrate as well as the capillary lumen, with minimal leukocytoclasis and endothelial swelling and without fibrinoid necrosis [Figures 2a and b]. Other laboratory investigations were within normal limits. The patient satisfied the American College of Rheumatology (ACR) classification criteria for cutaneous small vessel vasculitis.[4]

- (a) Skin biopsy from a papule on the upper arm of a patient with vortioxetine induced drug rash showing epidermis with focal erosions, dense neutrophilic clusters within the stratum corneum, and dermal inflammatory infiltrate (H and E, ×100); (b) Higher magnification of the same specimen showing endothelial swelling and neutrophils in the dermis and the capillary lumen with minimal leukocytoclasia (H and E, ×400).
Considering the absence of any other cause for the skin lesions and the improvement of dermatological manifestations on reducing the dose of the drug, a final diagnosis of vortioxetine induced cutaneous small vessel vasculitis was made. On discontinuation of the medication, there was complete resolution of the lesions with minimal scarring [Figure 3]. The Naranjo probability score was seven, suggestive of a probable drug reaction [Table 1].[5] She refused treatment with an alternate antidepressant.
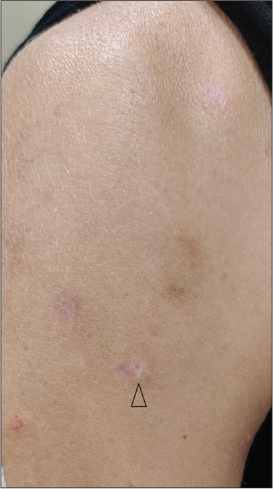
- Resolution of the pruritic crusted lesions with minimal scarring (arrow head), on discontinuation of vortioxetine.
| Naranjo adverse drug reaction probability scale | ||||
|---|---|---|---|---|
| Question | Yes | No | Do not know | Score assigned to the patient who manifested vasculitis following vortioxetine |
| Are there previous conclusive reports on this reaction | +1 | 0 | 0 | 0 |
| Did the adverse event appear after the suspected drug was administered? | +2 | −1 | 0 | +2 |
| Did the adverse reaction improve when the drug was discontinued or a specific antagonist was administered? | +1 | 0 | 0 | +1 |
| Did the adverse event reappear when the drug was re-administered? | +2 | −1 | 0 | 0 |
| Are there alternative causes (other than the drug) that could on their own have caused the reaction? | −1 | +2 | 0 | +2 |
| Did the reaction reappear when a placebo was given? | −1 | +1 | 0 | 0 |
| Was the drug detected in blood (or other fluids) in concentrations known to be toxic? | +1 | 0 | 0 | 0 |
| Was the reaction more severe when the dose was increased or less severe when the dose was decreased? | +1 | 0 | 0 | +1 |
| Did the patient have a similar reaction to the same or similar drugs in anyprevious exposure? | +1 | 0 | 0 | 0 |
| Was the adverse event confirmed by any objective evidence? | +1 | 0 | 0 | +1 |
| Total score | 7 | |||
Vortioxetine is an effective SSRI in MDD without the side effects associated with these groups of drugs, which include weight gain, sexual dysfunction, fatigue, and somnolence which make it the preferred antidepressant in younger adults. Since it is a newer drug, vortioxetine is not used widely and as a result, fewer side effects are reported.
Generalized pruritus is reported as a side effect of vortioxetine in 1–5% of the users.[3] Other dermatological side effects associated with vortioxetine are redness of face, neck, arms, and upper chest, red or purple spots on the skin and acneiform eruptions.[3,6] Dermatological side effects are observed with other serotonin reuptake inhibitors as well. They are attributed to either a hypersensitivity reaction to increased serotonin level in the blood or the resultant effect of increased activity of serotonin in epidermis and dermis.[7]
There are occasional reports of edema, petechiae, and ecchymosis of legs following vortioxetine in patients with normal platelet count, prothrombin time, and activated partial thromboplastin time.[3,8] However, since these reports are not supported with histopathological examination, the possibility of a drug induced vasculitis remains unknown in these cases. Table 2 shows the clinical profile of patients who had developed skin rash following vortioxetine, as documented in the literature.[3,6,8]
| Citing report | Age in years | Gender | Dose of vortioxetine at the time of appearance of rash | Time interval between onset of drug intake and appearance of rash | Morphology of rash* |
|---|---|---|---|---|---|
| Present report | 42 | Female | 10 mg | 8 months | Pruritic papule |
| Ay and Aytas | 20 | Female | 10 mg | 5 weeks | Acneiform eruption |
| Cetin and Kose | 43 | Female | 10 mg | 3 months | Edema, petechiae, and ecchymosis of legs |
| Okumus et al. | 34 | Female | 20 mg | 2 months | Itching, petechiae, and ecchymosis of popliteal fossa |
As per the ACR classification criteria, the presence of three of the following five features is needed to make a diagnosis of cutaneous small vessel vasculitis. (i) Age >16 years at disease onset; (ii) history of taking a medication at onset that may have been a precipitating factor; (iii) the presence of palpable purpura; (iv) the presence of a maculopapular rash; and (v) a biopsy demonstrating granulocytes around an arteriole.[4] The duration of 8 months between the onset of drug intake and the appearance of lesions in our patient was not against the diagnosis of drug induced vasculitis, since the interval between the onset of drug intake and the appearance of symptoms may range from hours to years in drug induced vasculitis.[9] Our patient manifested a popular rash and did not show any purpuric lesions. The ulceration of the papule and minimal scarring observed on healing could be attributed to the underlying cutaneous vasculitis. The exact nature of immunoreactants precipitating the vessel wall inflammation remains unknown in our patient since direct immunofluorescence microscopy was not carried out.
The skin biopsy in a suspected drug rash for the accurate diagnosis of the cutaneous small vessel vasculitis, as in our patient, is of paramount importance. Urticarial reactions and other life-threatening reactions have been reported to paroxetine, a related SSRI, but our patient may possibly represent the first reported case of cutaneous small vessel vasculitis to vortioxetine.[10]
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest
References
- The safety and tolerability of vortioxetine: Analysis of data from randomized placebo-controlled trials and open-label extension studies. J Psychopharmacol. 2016;30:242-52.
- [CrossRef] [PubMed] [Google Scholar]
- Serious dermatological adverse effects of vortioxetine: Two cases. Psychiatry Clin Psychopharmacol. 2018;28:355-7.
- [CrossRef] [Google Scholar]
- Cutaneous vasculitis In: Griffiths CE, Barker J, Bleiker T, Chalmers R, Creamer D, eds. Rook's Textbook of Dermatology (9th ed). Oxford: Wiley Blackwell; 2016. p. :102.1-38.
- [Google Scholar]
- A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239-45.
- [CrossRef] [PubMed] [Google Scholar]
- Acneiform eruption associated with the use of vortioxetine. Psychiatry Clin Psychopharmacol. 2019;29:226-8.
- [CrossRef] [Google Scholar]
- Itch and skin rash from chocolate during the fluoxetine and sertraline treatment: Case report. BMC Psychiatry. 2004;2:36.
- [CrossRef] [PubMed] [Google Scholar]
- A serious dermatological side effect due to vortioxetine: A case report. J Psychiatry Neurosci. 2020;33:87-91.
- [CrossRef] [Google Scholar]
- Cutaneous and systemic manifestations of drug-induced vasculitis. Ann Pharmacother. 2002;36:130-47.
- [CrossRef] [PubMed] [Google Scholar]
- Cutaneous effects of the most commonly used antidepressant medication, the selective serotonin reuptake inhibitors. J Am Acad Dermatol. 2007;56:848-53.
- [CrossRef] [PubMed] [Google Scholar]





