Translate this page into:
Hourglass shaped extragenital Csillag’s disease
*Corresponding author: Tiya Elizabeth John, Department of Dermatology, Venereology and Leprosy, Dr Somervell Memorial CSI Medical College and Hospital, Thiruvananthapuram, Kerala, India. tiyaelizabeth@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Tobin J, Samson JF, John TE, Thaj RR. Hourglass shaped extragenital Csillag’s disease. J Skin Sex Transm Dis. 2024;6:200-3. doi: 10.25259/JSSTD_8_2024
Abstract
Lichen sclerosus (LS) is a benign, chronic inflammatory dermatosis affecting both the epidermis and dermis. It commonly affects the anogenital area and is seen predominantly in women. In only 15–20% of cases, extragenital involvement is seen. In females, long-standing anogenital lesions progress to form hourglass or dumbbell or figure of eight morphology. Here, we are reporting a case of extragenital LS at an unusual site in an hourglass morphology, which is usually encountered in females. Our patient was started on ultrapotent topical corticosteroids, following which erythema was reduced. The patient is advised regular follow-up.
Keywords
Csillag’s disease
Extragenital Lichen sclerosus
Hourglass shape
INTRODUCTION
Lichen sclerosus (LS) was first described in 1887 by Hallopeau. It is a benign, chronic inflammatory dermatosis affecting the epidermis and dermis. LS most commonly affects the anogenital area (83% to 98%). In about 15–20% of cases, the extragenital sites are also involved1. It is known by many names, such as LS et atrophicus (LSA), Csillag’s disease, balanitis xerotica obliterans, kraurosis vulvae, and white spot disease.[1]
CASE REPORT
A 70-year-old male, with no known comorbidities, presented with a slowly increasing asymptomatic lesion over the left gluteal region for 8 years.
Dermatological examination revealed a single hourglass-shaped parchment-like atrophic plaque measuring 7 cm long, 4 cm wide at the widest and 2.5 cm wide at the narrowest part, with central erythema and surrounding hyperpigmentation over the left gluteal region [Figure 1]. Oral and genital mucosa was normal. A systemic examination revealed no significant findings. Clinically, morphea, LSA, and cutaneous T-cell lymphoma were considered as differentials.
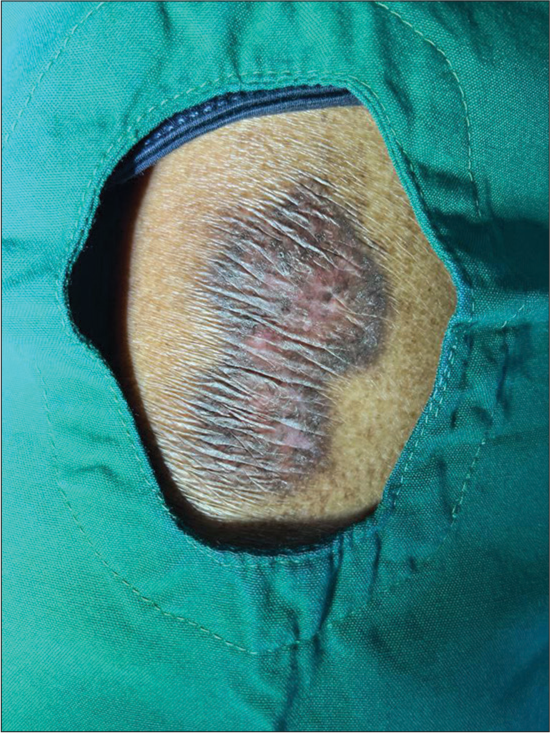
- Single hourglass-shaped parchment-like atrophic plaque measuring 7 cm long, 4 cm wide at the widest, and 2.5 cm wide at the narrowest areas with central erythema and surrounding hyperpigmentation over the left gluteal region.
The complete blood count, liver function tests, renal function tests, and thyroid function tests were within normal limits. Antinuclear antibody (ANA) testing by the enzyme-linked immunosorbent assay method was found to be negative.
Dermoscopy showed white structureless areas, follicular plugging, perifollicular scaling, and a few areas of hyperpigmentation [Figure 2].
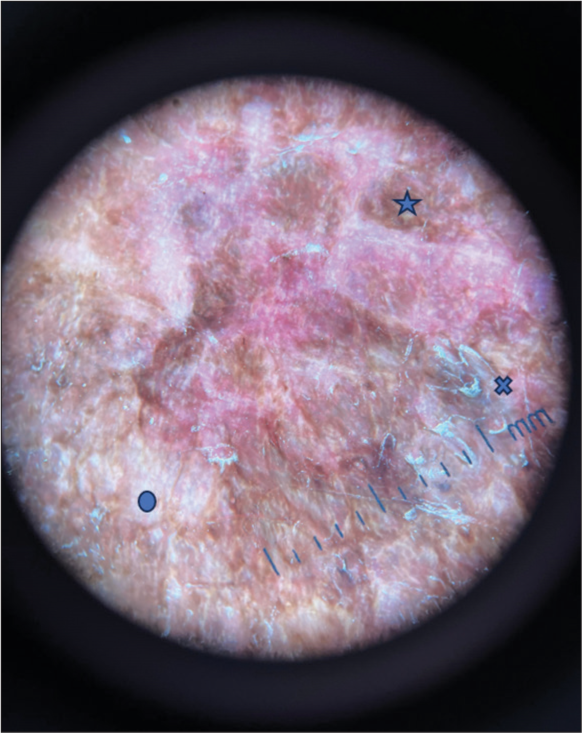
- Dermoscopy image showing white structureless areas (circle), perifollicular scaling (cross), hyperpigmentation (star) (Dermaindia M10A30).
Histological examination of the specimen showed hyperkeratosis, elongated rete ridges, basal cell vacuolar degeneration, and pigment incontinence in the epidermis. Inflammatory infiltrate composed of a band of lymphocytes and homogenization of collagen with mild edema in the dermis [Figure 3].
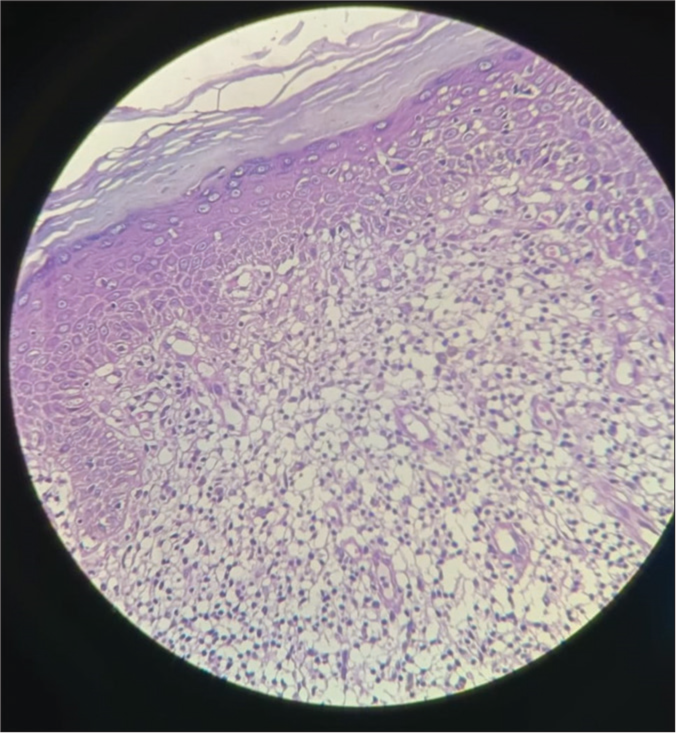
- Histopathology image showing hyperkeratosis, basal cell vacuolar degeneration, and pigment incontinence in the epidermis. In the dermis, inflammatory infiltrate is composed of a band of lymphocytes and homogenization of collagen with mild edema (Hematoxylin and eosin stain ×40).
Diagnosis-LSA.
The patient was started on Clobetasol Propionate 0.05% ointment twice daily application, and the patient was followed up at 2 weeks and 4 weeks after starting treatment. Following treatment, erythema and hyperpigmentation decreased [Figure 4]. The patient is advised regular follow-up.
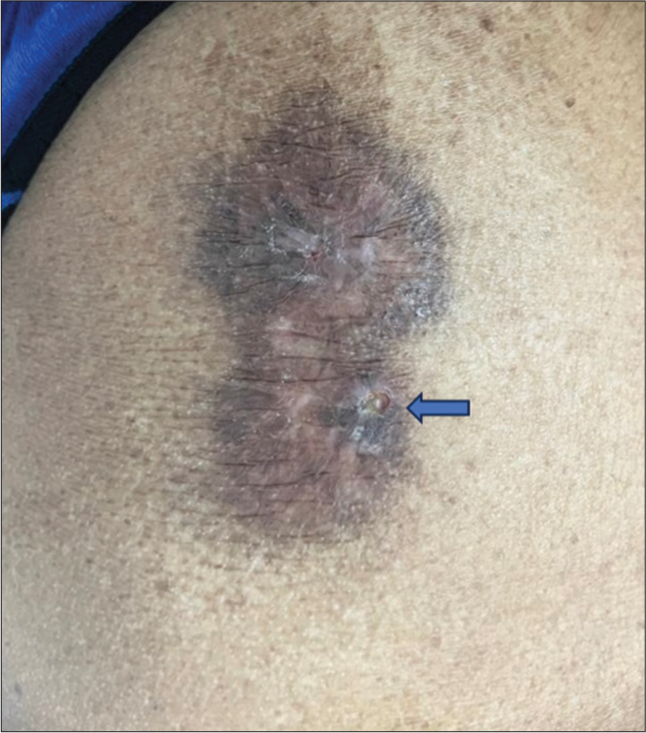
- Follow-up picture at 2 weeks, which shows reduced erythema and hyperpigmentation. Skin biopsy scar (arrow).
DISCUSSION
LSA is a rare chronic inflammatory dermatosis with genital and extragenital involvement. Women are most commonly affected F: M: 3:1–10:1.[2] Extragenital LS is seen only in 15–20% of LS patients and usually presents in middle-aged adults.[1] Genetic, autoimmune, infectious, environmental, and hormonal factors play a role in its etiology. A family history of LS in first-degree female relatives is seen in a few patients. LS is reported to be associated with human papillomavirus 16, hepatitis C, and the intake of drugs such as carbamazepine, imatinib, pembrolizumab, nivolumab, and ipilimumab. Autoimmune diseases such as Hashimoto’s thyroiditis, Graves’ disease, alopecia areata, systemic lupus erythematosus (SLE), Sjogren syndrome, and multiple sclerosis are observed in more than 25% of cases of LS and are more commonly reported in females.[1]
Clinical features
Vulvar LS presents as porcelain white atrophic papules coalescing into plaques, which can progress to sclerosis of the vulvar and perianal regions, which is described as a typical “figure of eight patterns,” “dumbbell shape,” or “hourglass shape.”[3]
Male LS presents as white porcelain-like scarring, resulting in phimosis. There may be tightening of the prepuce, leading even to meatal stenosis. Involvement of the prepuce is called posthitis xerotica obliterans, and involvement of the glans penis is termed balanitis xerotica obliterans.[3]
Extragenital LS, which is more common in females, is characterized by asymptomatic or pruritic ivory-white polygonal papules coalescing into plaques with a parchment-like appearance commonly affecting the thighs, neck, trunk, and lips.[3]
Investigations
Diagnosis is obtained by history-taking and clinical examination. Dermoscopy is used as an aid in the diagnosis. Histological examination is considered in cases with atypical characteristics or diagnostic ambiguity and is imperative when there is suspicion of neoplastic transformation. Routine blood investigations, along with an autoimmune workup, can be done if there is a suspicion of autoimmune associations.
Dermoscopy reveals structureless white to yellow areas, linear irregular vessels, comedo-like openings, perifollicular scaling, telangiectasias, rosettes, and chrysalis-like structures.[4]
Histopathology is characterized by (i) hyperkeratosis with follicular plugging, (ii) atrophy of stratum malpighii with hydropic degeneration of basal cells, (iii) pronounced edema and homogenization of collagen in the upper dermis, and (iv) an inflammatory infiltrate in the mid dermis.[5]
Differential diagnoses for genital LSA include lichen planus, lichen simplex chronicus, post-menopausal atrophy, erythroplasia of Queyrat, leukoplakia, squamous cell carcinoma, Paget’s disease, vitiligo, and child abuse.[3]
In the case of extragenital LS, discoid lupus erythematosus, morphea plaque type, lichen planus, vitiligo, hypopigmented mycosis fungoides, graft versus host disease, extramammary Paget disease, and squamous cell carcinoma are considered as differentials.[3]
Management
LS should be treated to prevent long-term complications, psychosexual impact, and the risk of malignant transformation. Ultrapotent topical corticosteroids are the first-line therapy. Clobetasol Propionate 0.05% is the most recommended treatment.[3] It is prescribed for twice-daily application until remission is achieved and then tapered. Clobetasol is reported to be superior to calcineurin inhibitors like tacrolimus and pimecrolimus. Calcineurin inhibitors such as tacrolimus 0.1% ointment or pimecrolimus 1% cream are prescribed as a twice or once daily application. Various other treatment modalities, such as phototherapy, photodynamic therapy using topical 5-aminolevulinic acid, and systemic retinoids, have been found to be effective.[3] Pulse therapy of high-dose corticosteroids combined with low-dose methotrexate has shown improvement in generalized extragenital LS refractory to conventional treatment.[6]
In long-standing lesions, scarring results in phimosis and urethral stricture, leading to complications such as urinary retention, anal stenosis, obstruction, and constipation, which are treated by surgical management.
Newer modalities include carbon dioxide laser, high-intensity focused ultrasound, and regenerative therapy using platelet-rich plasma.[7,8]
Since there is an absolute risk of 0.21–3.88% in women and 0.00– 0.91% in men for transformation to squamous cell carcinoma, all patients with LS are advised for long-term follow-up.[9]
CONCLUSION
LS is a rare disease, with genital LS being the most common type. Extragenital LS is rare, with more females affected, and is characterized by lesions affecting the neck, shoulder, and inner thighs. This case is reported for its extragenital presentation at an unusual location in a male patient.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
Dr. Joan Felicita Samson is on the editorial board of the Journal.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Diagnosis and treatment of lichen sclerosus: An update. Am J Clin Dermatol. 2013;14:27-47.
- [CrossRef] [PubMed] [Google Scholar]
- Lichen sclerosus-presentation, diagnosis and management. Dtsch Arztebl Int. 2016;113:337-43.
- [CrossRef] [PubMed] [Google Scholar]
- Fitzpatrick's dermatology (9th ed). New York: McGraw-Hill Education; 2019. Available from: https://www.accessmedicine.mhmedical.com/content.aspx?aid=1160945490 [Last accessed on 2024 Feb 29]
- [Google Scholar]
- Dermoscopic patterns in lichen sclerosus: A report of three cases. Indian Dermatol Online J. 2015;6:237-40.
- [CrossRef] [PubMed] [Google Scholar]
- Lever's dermatopathology: Histopathology of the skin United States: Lippincott Williams and Wilkins; 2022. p. :3866.
- [Google Scholar]
- Pulsed high-dose corticosteroids combined with low-dose methotrexate treatment in patients with refractory generalized extragenital lichen sclerosus. Arch Dermatol. 2009;145:1303-8.
- [CrossRef] [PubMed] [Google Scholar]
- Short-and long-term efficacy of focused ultrasound therapy for nonneoplastic epithelial disorders of the vulva. BJOG. 2017;124(Suppl 3):87-92.
- [CrossRef] [PubMed] [Google Scholar]
- Platelet-rich plasma for the treatment of lichen sclerosus. Plast Aesthet Res. 2021;8:63.
- [CrossRef] [PubMed] [Google Scholar]
- The risk of developing squamous cell carcinoma in patients with anogenital lichen sclerosis: A systematic review. Gynecol Oncol. 2020;157:671-7.
- [CrossRef] [PubMed] [Google Scholar]







