Translate this page into:
Iatrogenic Cushing’s syndrome, cataract, and metabolic syndrome in an adult following topical steroid abuse for dermatophytosis
*Corresponding author: Ramesh M. Bhat, Department of Dermatology Venerology and Leprosy, Father Muller Medical College, Mangaluru, Karnataka, India. rameshderma@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Satish S, Kiran, Bhat RM, Jose A. Iatrogenic Cushing’s syndrome, cataract, and metabolic syndrome in an adult following topical steroid abuse for dermatophytosis. J Skin Sex Transm Dis. 2024;6:160-2. doi: 10.25259/JSSTD_46_2024
Abstract
A much is known about the cutaneous side effects of topical corticosteroids (TCs) often neglecting their potential systemic adverse events, including Cushing’s syndrome (CS). Although cases of CS following TCs abuse are a well-documented entity in the pediatric population, similar instances in adults are less common and less discussed. Here, we describe a case of dermatophytosis where the patient developed multiple systemic adverse effects, including significant weight gain, numerous skin striations, and blurred vision, as a result of TCs misuse complicating the otherwise non-significant disease. Hence, it is crucial to emphasize the importance of consulting a dermatologist instead of relying on easily accessible over-the-counter medications from pharmacies, which many people in the general population often resort to.
Keywords
Cushing’s syndrome
Topical steroid abuse
Over the counter steroid
Clobetasol propionate
Dermatophytosis
INTRODUCTION
Topical corticosteroids (TCs) are widely prescribed in dermatology for their potent anti-inflammatory effects. However, prolonged and unsupervised use can lead to severe cutaneous and systemic side effects. Dermatophytosis, a common skin infection, often falls prey to TCs abuse due to their low cost and easy availability over the counter (OTC). The uninhibited dispensing of corticosteroid-mixed creams, combined with a lack of awareness about potential side effects, poses significant challenges in treating steroid-modified cases.
CASE REPORT
A 38-year-old male presented with red, itchy, flaky, and raised lesions covering his body for the past 2 years. He also reported excessive weight gain, multiple skin striations, and blurred vision. His medical history revealed that he is diagnosed to have hypertension for 2 years. Further inquiry revealed that he had been using an OTC cream called Dermi 5 for the past 2 years (containing Clobetasol Propionate 0.05% weight per weight [w/w]), Gentamicin 0.1% w/w, Tolnaftate 1%,w/w Iodochlorhydroxyquinoline 1%w/w, and Clotrimazole 1% w/w) intermittently for quick relief from itching, with no other systemic treatment for same.
On physical examination, he had a moon-like face and central obesity with a body mass index of 32.79 kg/m2 [Figure 1]. His blood pressure readings were consistently above 150/100 mmHg. Cutaneous examination revealed multiple striae rubra over the axilla, groin, and trunk, a few acneiform eruptions, and ill-defined erythematous scaly papules and plaques. Dermoscopic examination showed diffuse erythema, peripheral white scales, and serpentine branching vessels [Figure 2]. A potassium hydroxide mount demonstrated fungal filaments, confirming dermatophytosis. Further evaluation revealed abnormal fasting blood glucose levels at 225 mg/dL (70–99), elevated triglycerides at 200 mg/dL (<150), and a low 6 a.m. serum cortisol level of 22.4 nmoL/L (123–626). Due to financial constraints, the adrenocorticotropic hormone stimulation test could not be conducted. The patient was diagnosed with exogenous Cushing’s syndrome (CS), hypothalamic-pituitary-adrenal (HPA) axis suppression, and metabolic syndrome. A detailed ophthalmological evaluation also diagnosed steroid-induced cataract.
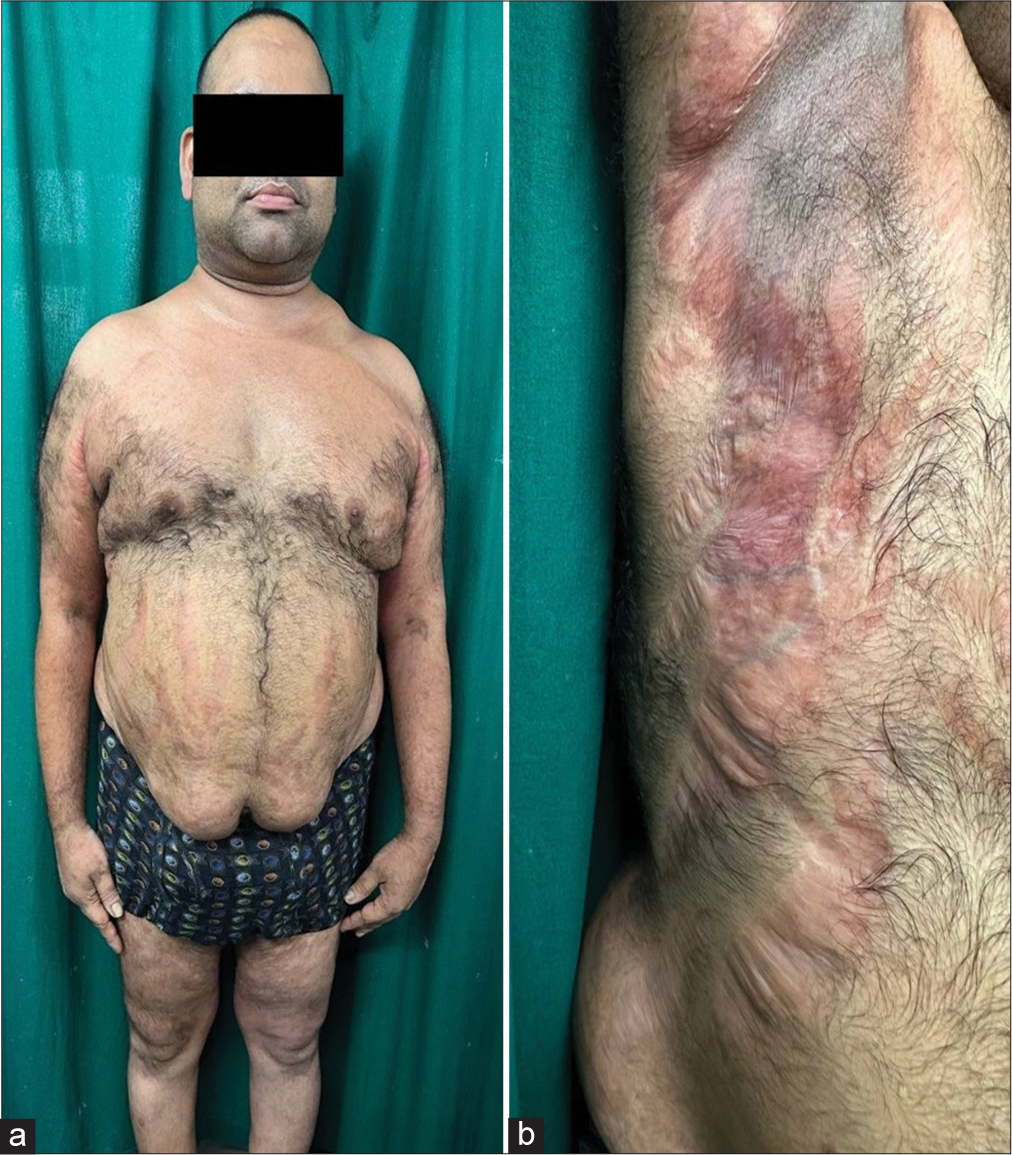
- (a): Clinical image of the patient showing moon-like face, truncal obesity; and (b): multiple striae rubrae.
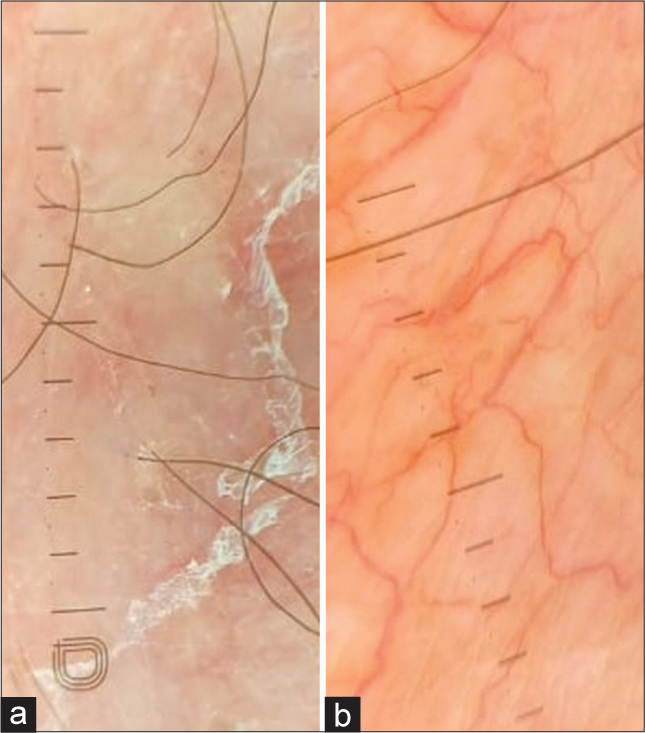
- (a): Dermoscopy showing diffuse erythema, white peripheral scales, DermLite DL5, ×10 non-polarized; (b): Serpentine and branching vessels (DermLite DL5, ×10, polarized).
The patient was advised to discontinue the use of TCs and initiated on itraconazole 200 mg weekly and luliconazole lotion for the treatment of dermatophytosis. In addition, a tapering dose of prednisolone 5 mg was started. For the elevated blood glucose levels, the patient was started on metformin 500 mg combined with teneligliptin 20 mg. On follow-up, the patient exhibited a reduction in Cushingoid features and improvement in the dermatophytosis infection.
DISCUSSION
TCs are widely used in treating inflammatory dermatological conditions, and when used properly, they can significantly improve prognosis. However, misuse of TCs is known to result in various cutaneous and systemic adverse events. Cutaneous adverse events include acneiform eruption, cutaneous atrophy, rosacea, telangiectasia, hypopigmentation, striae, perioral dermatitis, and hypertrichosis; systemic adverse events include exogenous CS, suppression of the HPA axis, hypertension, dyslipidemia, failure to thrive, glaucoma, and cataracts.[1,2]
While cases of exogenous CS following TCs abuse are well-documented in children due to their larger body surface area, similar side effects in adults, though less common, are not unheard of.[3,4] Our patient had developed both cutaneous and systemic side effects in the form of acneiform eruptions, striae, atrophy, exogenous CS, steroid-induced cataract, and metabolic syndrome.
In a developing country like India, TCs are easily accessible at most pharmacies and retail stores and are often dispensed OTC. The affordability, widespread availability, and lack of knowledge about the side effects of these drugs have contributed to their misuse.
To combat this growing issue, various measures need to be implemented. The Indian Association of Dermatologists, Venereologists, and Leprologists, with support from the International League of Dermatological Societies and the International Society of Dermatology succeeded in including topical steroids in Schedule H of the Drugs and Cosmetics Act from November 1, 2018.[5] Besides these regulations, increasing public awareness about the risks of topical steroid misuse and the necessity of consulting a dermatologist for skin concerns is crucial.
CONCLUSION
TCs are among the most frequently used medications in dermatology. This case highlights the serious systemic side effects of long-term TC abuse. It emphasizes the need for stringent regulations and public awareness to prevent the misuse of these drugs and encourages seeking professional dermatological care for skin issues.
Ethical approval
The research/study approved by the Institutional Review Board at Father Muller Institutional ethics committee, number ECR/540/Inst/KA/2014/RR-20, dated August 13, 2024.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
Dr. Ramesh M. Bhat is on the editorial board of the Journal.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Topical corticosteroids abuse: A clinical study of cutaneous adverse effects. Indian J Dermatol. 2017;62:675.
- [CrossRef] [PubMed] [Google Scholar]
- Topical-steroid-induced iatrogenic Cushing syndrome in the pediatric age group: A rare case report. Indian J Endocrinol Metab. 2013;17:S257-8.
- [CrossRef] [PubMed] [Google Scholar]
- Topical corticosteroid-induced iatrogenic cushing syndrome in an infant; A case report with literature review. Ann Med Surg (Lond). 2021;71:102978.
- [CrossRef] [PubMed] [Google Scholar]
- Cushing syndrome in a pediatric patient with topical steroid overuse. Case Rep Endocrinol. 2022;2022:8487737.
- [CrossRef] [PubMed] [Google Scholar]
- Steroid skin creams only on prescription. 2018. The telegraph online;. Available from: https://www.telegraphindia.com/india/steroid-skin-creams-only-onprescription/cid/1344070 [Last accessed on 2024 Sep 03]
- [Google Scholar]







