Translate this page into:
Dupilumab: Evaluating its role in atopic dermatitis, prurigo nodularis, eczemas, urticaria, alopecia areata and vesiculobullous disorders
*Corresponding author: Aditya Kumar Bubna, Department of Dermatology, Katihar Medical College, Katihar 854 106, Bihar, India. zimbabwa21@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Bubna AK, Viplav V. Dupilumab: Evaluating its role in atopic dermatitis, prurigo nodularis, eczemas, urticaria, alopecia areata and vesiculobullous disorders. J Skin Sex Transm Dis. 2024;6:113-25. doi: 10.25259/JSSTD_45_2024
Abstract
Dupilumab is a fully human monoclonal IgG4 antibody that targets IL-4 and IL-13 signaling pathways. It is approved by the US-FDA for the treatment of atopic dermatitis and prurigo nodularis. Besides, it has shown efficacy in various off-label dermatologic conditions. This review will elaborate on the utility of dupilumab in atopic dermatitis, prurigo nodularis, eczemas, urticaria, alopecia areata and vesiculobullous disorders.
Keywords
Dupilumab
Atopic dermatitis
Prurigo nodularis
Vesiculobullous disorders
Alopecia areata
INTRODUCTION
Dupilumab is a fully human monoclonal immunoglobulin 4 (IgG4) antibody, which has been approved by the United States Food and Drug Administration for the treatment of moderate-to-severe atopic dermatitis (AD) in adults/pediatric patients (>6 months of age), and prurigo nodularis (PN).[1,2] Besides, it has also shown efficacy in various off-label dermatologic conditions. The aim of this narrative review is to describe the utility of dupilumab in approved dermatoses as well as its implication in off-label dermatologic disorders.
PHARMACOKINETICS
Following a 600 mg subcutaneous (SC) injection of dupilumab, maximum serum concentrations are attained within 7 days, with steady state concentrations available by week 16. Bioavailability of dupilumab is 64% and its t1/2 ranges from 11.7 to 20.5 days.[3,4] Being a human monoclonal IgG4 antibody, degradation of dupilumab occurs through catabolic pathways into small peptides similar to endogenous IgG. It is considered that following degradation, elimination occurs through the renal route.[3]
ADMINISTRATION AND DOSING
Dupilumab is administered subcutaneously. The recommended dose is 300 mg every fortnight, after an initial loading dose of 600 mg (given as two 300 mg injections at different sites).[3]
However, some studies suggest weekly administration of 300 mg of dupilumab to be equally or even more effective than the current approved dosing schedule.[5,6]
METHODS
PubMed, MEDLINE, and Embase databases were searched from the period January 1, 2014, to March 15, 2024. Searched terms were “dupilumab” or “Dupixent” in combination with the following: “atopic dermatitis,” “prurigo nodularis,” “chronic hand eczema (CHE),” “allergic contact dermatitis (ACD),” “alopecia areata (AA),” “chronic urticaria,” “bullous pemphigoid (BP),” “pemphigoid gestationis (PG),” “Brunsting Perry pemphigoid (BPP),” “epidermolysis bullosa pruriginosa (EBP),” “Grover’s disease (GD),” “Hailey-Hailey disease (HHD),” “pemphigus vulgaris (PV),” “lichen planus (LP),” “lichen planus pemphigoides (LPP),” “Kimura’s disease (KD),” “chronic pruritus,” “chronic actinic dermatitis (CAD),” “cutaneous T-cell lymphoma,” “Well’s syndrome (WS),” “keloids,” “hypertrophic scars (HTS),” “granuloma annulare (GA),” “reactive perforating collagenosis (RPC),” “lichen amyloidosis (LA),” “eosinophilic annular erythema (EAE),” “Netherton’s syndrome (NS),” “papuloerythroderma of ofuji (PEO),” “hidradenitis suppurativa)HS),” “hyperimmunoglobulin-E syndrome (HIES),” “neurofibromatosis (NF),” “IPEX syndrome,” “palmoplantar pustulosis (PPP),” “hypereosinophilic syndrome (HES),” “lamellar ichthyosis (LI),” “graft versus host disease (GVHD),” “food allergy,” and “trichothiodystrophy (TTD).” All original studies including case reports, case series, and clinical trials were included, if they were full text, involved a dermatologic condition treated by dupilumab, and published in English. Articles were excluded, if they were conference abstracts, non-clinical reports or in vitro studies. All references were checked and additional articles included in the study. Schematic representation of the search strategy is delineated in Figure 1.
- Schematic representation of the search strategy followed for this review.
RESULTS
Clinical uses
AD
Profitability of dupilumab in AD was first exemplified in a robust, early-phase, development program by Beck et al.[7] At 12 weeks, 85% of dupilumab treated patients achieved 50% reduction of the eczema area severity index (EASI) score compared to 35% in the placebo group (P < 0.001). Similarly, the pruritus numeric rating scale (NRS) score reduced by 55.7% for dupilumab treated patients, compared with 15.1% for the placebo group.
A larger, dose-ranging trial of 380 patients using five dupilumab dosing schedules with one placebo, showed 300 mg weekly and biweekly dosings to be more effective than the 300 mg monthly, 200 mg biweekly, and 100 mg monthly dosing schedules; with percentage reduction of the EASI being 73.7%, 68%, 64%, 65%, and 45%, respectively. The placebo arm on the other hand depicted reduction of the EASI score by 18.1% (P < 0.0001).[8]
Dupilumab’s efficacy in AD was further corroborated by two phase III studies; SOLO-1 and SOLO-2. In both programs, 1379 adult patients with moderate-to-severe AD were randomized to receive placebo or a 600mg loading dose of dupilumab, followed by weekly or biweekly injections of dupilumab. In SOLO-1, Investigator’s Global Assessment (IGA) score of 0 or 1 with a 2-point or greater improvement was reached by 37% in the weekly cohort, and 38% in the fortnightly cohort, with EASI-75 responses being achieved by 52% and 51% in both groups, respectively. In the placebo group, IGA score was sustained by 10%, and EASI-75 response was seen in 15% of the patients. In SOLO-2, both dupilumab groups achieved a 36% IGA response, with EASI-75 responses observed in 48% of patients receiving weekly dupilumab, and 44% of patients receiving fortnightly dupilumab. The placebo group on the other hand demonstrated IGA and EASI-75 responses in 8% and 12% of patients, respectively.[4]
An additional phase III trial, CHRONOS, analyzed concomitant topical corticosteroid (TCS) along with dupilumab versus placebo, over 52 weeks in moderate-to-severe AD. The EASI-75 response was obtained by week 16, and sustained through 1-year in 64% of patients treated with dupilumab (300 mg weekly) plus a TCS; and in 65% of patients receiving dupilumab (300 mg fortnightly) plus a TCS. In the placebo group, the EASI-75 response at 1 year was reported in 22% of patients.[9] Other findings revealed through this study included; fewer AD flares in both dupilumab groups (13% and 14% in those receiving it weekly and fortnightly, respectively, compared with 41% of those treated with placebo), reduction in TCS and systemic rescue therapy with 33.7% (weekly dupilumab) and 30.1% (fortnightly dupilumab) of topical and systemic medication free days versus 23.7% in the placebo group (P < 0.0001), statistically significant improvement in the pruritis NRS score at week 2 in the dupilumab plus TCS groups versus placebo, with improvements maintained through week 52, and parallel improvement in health-related quality of life (QoL) and least square mean reduction from baseline in the dermatology life quality index (DLQI) scores of each group, along with statistically significant benefit in both dupilumab treated groups (P < 0.0001).[9]
Moreover, the safety profile of dupilumab according to these trials (SOLO-1, SOLO-2, and CHRONOS) was comparable to placebo, with discontinuation rates being lower overall for dupilumab versus placebo (3% vs. 8%, respectively).
Mechanism of action of dupilumab in AD is described in Figure 2.[7,10] Dupilumab also significantly decreases serum levels of the chemokine CCL17, a key regulator of Th2 mediated immunity; that is a specific and objective marker of AD disease activity.[11]
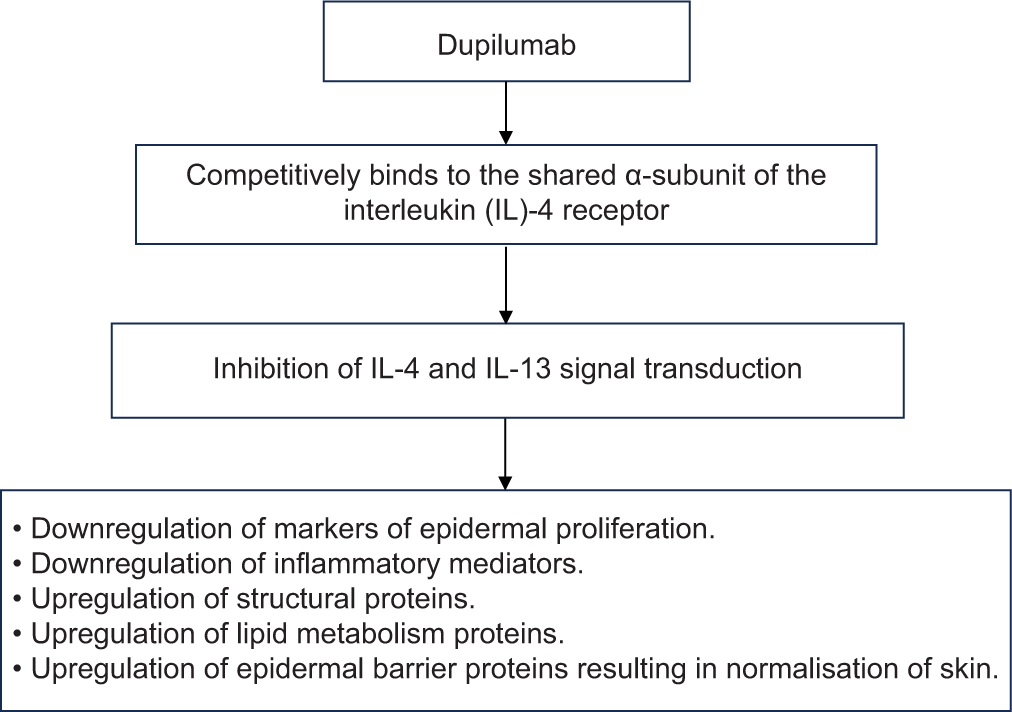
- Mechanism of action of dupilumab in atopic dermatitis.
PN
PN is a chronic cutaneous disorder presenting as multiple pruritic nodules particularly involving the trunk and extensor surfaces of the extremities. In 50% of cases, an atopic predisposition is present.[12] Although, the pathogenic mechanism underlying PN is not clearly elucidated, an emerging role of Th2 signaling is suggested. Increased expression of phosphorylated signal transducer and activator of transcription (STAT) 6, (a key transcription factor mediating Th2 signaling), as well as upregulation of interleukin (IL)-31, IL-4, and IL-13 in PN affected skin, majorly contribute toward the intense pruritus accounted in PN by activating sensory neuronal pathways following direct stimulation of Th2 inflammatory factor receptors in the sensory neurons of the dorsal root ganglia.[13-15] Dupilumab by binding to IL-4Rα (type I and II) and IL-13Rα1 receptors, antagonizes Th2 cell-mediated inflammatory responses and blocks significant abnormal interactions and dysfunction between immune cells and neuronal circuits that drive the itch-scratch cycle in PN.[7,16]
Till date, around 120 PN patients with successful outcomes following dupilumab therapy(label dosing) in individual reports and case series have been reported and are outlined in Supplementary Table 1.[16-36] Pruritus is generally the first symptom to respond in PN, followed by nodular regression. Besides, latency periods for the reduction of pruritus and clinical manifestations of PN vary from patient to patient. Further in atopics with PN, a faster response is obtained, although final outcomes in atopics and non-atopics are similar.[7,17]
In a systematic review by Husein-ElAhmed et al.,[37] the authors concluded that following dupilumab therapy complete remission can be expected if by 8 weeks patients observe clinical improvement, and a 50% reduction of lesions on the NRS. Conversely, if amelioration of lesions occurs after 12 weeks and a 50% reduction of lesions is not achieved, obtaining a complete clinical response is unlikely.
CHE
In CHE, clinical manifestations are diverse, and pose diagnostic/therapeutic challenges. Further, the debilitating quality of CHE profoundly affects the QoL of patients.[38] Frequent phenotypes observed include dyshidrotic (vesicular) and hyperkeratotic/fissured hand eczema, allergic contact dermatitis (ACD), and irritant contact dermatitis.[39] As IL-4R is highly elevated in CHE skin, IL-4/IL-13 may have a role in the pathophysiology of CHE, similar to that observed in AD. This imbrication of CHE and AD transcriptomes outlines the efficacy of dupilumab in CHE.[40] At present, utility of dupilumab in CHE has been majorly demonstrated in case reports/case series, with two prospective observational studies and one retrospective review highlighting the same [Supplementary Table 2].[41-54]
ACD
ACD is commonly seen in patients with AD, suggesting a type IV hypersensitivity reaction, associated with defective skin barrier function. Although Th1 and Th17 cells are traditionally considered the primary effector cells in ACD, recent studies have demonstrated cytokine responses to be hapten-specific in ACD, with additional elaboration of Th2 and Th22 responses, depending on the involved allergen.[55] Besides, after prolonged/repeated allergen exposure in ACD, a predominant Th2 response is elicited.[56]
The role of IL-4 in ACD has been substantiated by IL-4 knockout mice to contact allergens like oxazolone, characterized by Th2 mediated sensitization.[57] In addition, immunochemical analysis in ACD patients have revealed IL-4 receptor α-chain immunoreactivity and >5-fold increase in IL-4 mRNA levels, thereby concluding IL-4 and IL-13 as important cytokines in ACD.[58,59] By targeting IL-4, dupilumab can reduce symptoms in this subset of patients. Moreover, as barrier function improves in patients with AD following dupilumab therapy, patients susceptibility to allergens further reduce.[60] Besides, even in ACD not associated with AD, dupilumab maybe effective following failed immunosuppressive therapies.[61]
Dupilumab has been particularly beneficial in ACD secondary to fragrances, balsam of Peru, sesquiterpene lactones, nickel, glues, textile, rubber, dyes, cosmetic preservatives, and home care products.[62-67] Further, in systemic allergic dermatitis, namely, balsam and nickel allergy syndrome, where ACD remains uncontrolled despite prudent contact allergen avoidance, dupilumab has proven to be an efficacious solution.[68] However, even when on dupilumab, localized flares of ACD have been reported, necessitating simultaneous allergen avoidance. In addition, in patients experiencing good clinical response with dupilumab, a trial of re-exposure to contact allergens needs to be considered, and though dupilumab is effective in some cases of ACD, clinicians need to be aware that patients may not achieve complete clinical improvement, and may benefit from shorter intervals/longer duration of follow up; use of standardized assessments to monitor for clinical improvement; and continued allergen avoidance.[69] Another point to consider is the occurrence of false-negative patch test reactions and recall dermatitis at patch test sites in patients on dupilumab for ACD. For this, repeat patch testing is warranted once dupilumab is discontinued. Moreover, patch testing in patients on dupilumab should always be interpreted with caution.[70] At present, literature emphasizing the utility of dupilumab is limited to case reports/series with the need for randomized controlled trials (RCTs) to provide more evidence in this regard.
AA
AA is an inflammatory non-scarring alopecia commonly encountered by dermatologists. Despite its increased prevalence, therapeutic outcomes of AA remain limited. Based on the fact that AA and AD demonstrate common inflammatory signaling pathways (including Th2 overactivation; and upregulation of IL-4 and IL-13), the role of dupilumab in AA has been proposed.[71,72] However, antagonistic Th2 effects by dupilumab results in dysregulation (either up/downregulation) of Th1/Th17 responses, causing a cytokine shift that eventually determines whether dupilumab will precipitate or remit AA.[73] Moreover, in patients with severe AD and AA, where massive release of interferon (IFN)-α is involved, control of AD is sufficient to allow resolution of AA with dupilumab. Conversely, in patients without pre-existing AA in AD, dupilumab therapy heralds an immune shifting that paradoxically precipitates AA, mediated by autoreactive CD8+ T-cells.[74] In addition, it is seen that reduction in serum levels of thymus and activation-related chemokine (TARC) is associated with hair regrowth in AA, whereas elevation of TARC levels exacerbates AA following treatment with dupilumab.[75] Furthermore, blockade of IL-4 and IL-13 by dupilumab disrupts the interleukin milieu in sebaceous glands, resulting in sebaceous gland atrophy and non-scarring alopecia,[76] which histologically does not express features of AA, suggesting dupilumab-induced non-scarring alopecia to be an AA-like drug reaction.[77] Interestingly, Ständer et al.,[78] have postulated that dupilumab neither induces nor improves AA, with the observed association being random, due to high rates of spontaneous remission and IFN signature characterization in AA. Nevertheless, as reports exemplify both precipitation and alleviation of AA post dupilumab therapy, studying them become essential and are elaborated in Supplementary Table 3.[73,75,76,78-90]
Chronic urticaria
Chronic urticaria is a mast cell (MC) mediated disease presenting as wheals, angioedema or both, and lasting for >6 weeks. Although current management guidelines recommend a step-wise approach, beginning with antihistamine therapy and gradually escalating its dose, some patients remain refractory to the usual treatment guidelines, that include omalizumab and cyclosporine.[91] Dupilumab is of particular value in those chronic urticaria patients who are unresponsive to omalizumab, in both chronic spontaneous urticarial (CSU) and chronic inducible urticaria.[92-102]
Th2 inflammation dominates urticaria pathogenesis, wherein IL-4 and IL-5 stimulate the production of IgE antibodies that subsequently bind to the FcεRI, and promote MC degranulation. Further, IL-4 augments FcεRI cell surface expression, FcεRI α-specific mRNA, and FcεRI -mediated histamine release. In addition, following long-term exposure, IL-4 alters human skin MCs by strengthening MC numbers and intensifying the processes associated with allergic inflammation. By blocking both IL-4 and IL-13, dupilumab halts the class switching of B-cell immunoglobulins toward IgE and ameliorates symptoms and signs associated with urticaria.[97,103-105]
In two RCTs that studied the efficacy of dupilumab in CSU for omalizumab naïve patients (n = 138) and omalizumab intolerant/incomplete responders (n = 108), it was seen that in the omalizumab-naïve group, there was an 8.5 points higher reduction in the urticaria activity score over 7 days (UAS7) (P = 0.0003), as well as greater reduction of pruritus and wheals at week 24 (31.4% of patients), whereas in the omalizumab refractory group, the UAS7 score had reduced to zero in 13% of patients treated, and improvement in itch and hives severity demonstrated only a marginal statistical significance based on health authority hierarchy.[106] Apart from the above RCTs, remaining data for dupilumab in chronic urticaria are limited to case reports/series [Supplementary Table 4].[93-98,101,102]
BP
BP is the most common autoimmune subepidermal blistering disease of the skin, affecting all age groups, though most commonly encountered in the elderly.[107] Two target antigens; BP180 and BP230 are involved in blister formation in BP. In addition, type 2 pro-inflammatory cytokines, namely, IL-4 and IL-13 have been detected in blister fluid/skin biopsies of BP patients, and increased levels of IgE and peripheral eosinophilia correlate with disease activity.[108,109] Dupilumab in BP directly blocks IL-13 and IL-4 and indirectly downregulates IgE secretion and eosinophilic activity by inhibiting preactivated B-cell proliferation; directing human B-lymphocytes to switch to IgG4, and plummeting eosinophilic chemotaxis and Th2-associated chemokine activity without significant modulation of Th1-associated genes.[110,111] Besides, dupilumab reduces peripheral itch sensory neuronal signaling by antagonizing IL-4, IL-13, and reducing IL-31 secretion from eosinophils and is of particular value in BP.[112]
Dupilumab is effective in new/relapsed cases of BP, requiring at least 16–20 weeks to demonstrate disease clearance (defined as absence of both bulla and pruritus), or satisfactory response (defined as documented clinical improvement and patients desire to continue dupilumab).[113,114] In general, within 5 weeks, the consolidation phase (time at which no new lesions have developed for a minimum of 2 weeks and approximately 80% of lesions have healed) ends, with significant improvements being witnessed after 8 weeks of treatment initiation.[114] Pruritus is the first symptom to improve (as early as 1 week) majorly improving the patient’s QoL.[115] Furthermore, dupilumab enables amelioration of blistering and intractable pruritus in those BP patients refractory to systemic glucocorticoids, multiple immunosuppressants, omalizumab, intravenous immunoglobins (IVIG), rituximab, and plasmapheresis.[116,117] Moreover, dupilumab can be combined with systemic glucocorticoids, azathioprine, mycophenolate mofetil (MMF), methotrexate, IVIG, and rituximab to treat BP, enabling faster steroid taper, quicker dose reduction, and subsequent stoppage of immunosuppressants.[107,113,114,116-119] Interestingly, Yang et al.,[120] demonstrated combination of dupilumab and low-dose glucocorticoids (0.4mg/kg/day methylprednisolone) to be effective/safe in treating BP, allowing the cumulative dose of methylprednisolone to be >50% less than the conventional regimen and offering an added steroid sparing advantage.
In addition, dupilumab delineates efficacy/safety in treating immune checkpoint inhibitor (ICI)-induced BP. Of the ICIs that induce BP, dupilumab has been effective against nivolumab and pembrolizumab.[121,122] As traditional therapies such as azathioprine and MMF are relatively contraindicated in these patients due to their concurrent malignancies, dupilumab serves as a valuable alternative due to lower rates associated with immune suppression.[122] Cessation of new blister formation is usually evident by 1–2 months; following dupilumab treatment in these cases.[121,122] Besides, dupilumab is safe as well as effective in elderly individuals and adolescents with refractory BP.[107,113-119] Even so, as dupilumab has minimal immunosuppressive effects, it can be safely used in patients with tuberculosis and hepatitis C.[115,123] In BP presently, the dose of dupilumab has not been standardized. In most patients, the AD dosing is employed. However, in patients not showing satisfactory responses, the dose can be augmented by 300 mg weekly or 600 mg every other week. This updosing has been associated with clinical remission with no apparent adverse reactions.[113,120]
Furthermore, dupilumab’s favorable discourse has been observed in BP variants such as vesicular pemphigoid, erythrodermic pemphigoid, and pemphigoid nodularis.[123-125] At present, the utility of dupilumab in BP is only exemplified in case reports/series, with the need of RCTs to strengthen each observation.[107,113-120]
PG
PG is a dermatosis of pregnancy presenting in the second and third trimester. Despite spontaneous improvement during late pregnancy, relapses during the time of delivery are typical. Recurrence in successive pregnancies is frequent; occasionally having an earlier onset that may pose the risk of prematurity and growth restriction for the fetus.[126] Due to clinical and pathogenic similarities of PG and BP, dupilumab’s utility in PG is considered.
Dupilumab (label dosing) in an anecdotal report demonstrated improvement of pruritus and bullous lesions in a 30-year-old woman with PG (20 weeks of gestation), along with prednisolone. Besides, following delivery, there was no recurrence in the post-partum period, and within 3 months BP-180 antibodies had negativized. Even so, following the loading dose of dupilumab, only two maintenance doses were sufficient to control the disease. Moreover, no untoward events were witnessed in the mother and neonate.[127]
BPP
BPP is a clinical subtype of cicatricial pemphigoid (CP) without mucous membrane involvement; characterized clinically by subepidermal blisters, erosions, hemorrhagic crusts, and atrophic scars confined to the head, neck, and upper trunk.[128] Dupilumab outlined favorable effects in two patients with BPP, unresponsive to conventional therapy (that included systemic steroids, MMF, methotrexate, dapsone, doxycycline, nicotinamide, and rituximab). Rapid improvement and clearance of lesions were observed after the completion of three doses of dupilumab.[129,130] As pathogenesis in BPP comprises lymphocytosis, eosinophilia, elevated levels of basement membrane IgG, and tissue scarring/fibrosis, the role of dupilumab in BPP has been propounded.[129]
EBP
EBP is a rare clinical subtype of autosomal dominant (or less commonly recessive) dystrophic epidermolysis bullosa (DEB). In addition to usual manifestations of DEB such as skin fragility (secondary to trauma), milia, and nail dystrophy, EBP presents with severe pruritus, prurigo-nodularis, and lichen simplex chronicus such as lesions, often complicating the clinical diagnosis.[131] Proposed mechanism of dupilumab in EBP is blockade of IL-4Rα-induced sensitization of sensory neurons to pruritogens, thereby interrupting the itch-scratch cycle, a critical component in the recovery of lesions in EBP.[14] However, expressions of IL-4 and IL-13 in EBP remain to be investigated. Nevertheless, as dupilumab has been successful in the treatment of a variety of pruritic diseases, dupilumab maybe considered a good choice in EBP.[132] At present, only case reports/series have elaborated the usefulness of dupilumab in EBP and are portrayed in Table 1,[132-135] with RCTs being needed to strengthen these findings.
| S. No. | Authors/Year | Study type | Patient details | Previous failed therapies | Dupilumab therapy | Remarks | Adverse effects |
|---|---|---|---|---|---|---|---|
| 1. | Shehadeh et al.,[133]/2020 | Case report. | 52-year-old female with blisters and erosions since birth. | • Super potent TCS. • Fexofenadine. • Hydroxyzine. • Promethazine. • Medicinal cannabis. • St John’s Wort. |
Label dosing. | • At 4 weeks: Marked improvement seen. • At 12 weeks: Improvement progressed. |
None. |
| 2. | Clawson et al.,[134]/2021 | Case report. | 39-year-old male with papulonodules on bilateral upper and lower limbs. | • TCS. • Topical calcineurin inhibitors. • Oral dapsone/cyproheptadine. |
Label dosing. | • Complete resolution of pruritus by week 4 of dupilumab treatment. • No development of new lesions. • At 9 months there was complete relief of itching with slowly resolving skin lesions. |
None. |
| 3. | Zhou et al.,[135]/2020 | Case series (2 patients). | 15-year-old male and 27-year-old female with unresponsive EBP. | • Systemic steroids. • Cyclosporine. • Mycophenolate mofetil. • Thalidomide. • Lenalidomide. • Omalizumab. • Tofacitinib. • Ondansetron. • N-acetylcysteine. • Gabapentin. • Pregabalin. • Naltrexone. • Melatonin. • Clonidine. • Antidepressants • Anti-histaminics. • Phototherapy. |
Label dosing in one patient and 300 mg/week in another patient. | • Patient receiving label dosing: At 6 weeks reduction in itch from 8/10 to 4.5/10 in the ten-point itch scale. • Patient receiving 300mg weekly dose showed reduction in itch from 10/10 to 2/10 in the 10-point itch scale. |
None. |
| 4. | Wang et al.,[132]/2021 | Case report. | 10-year-old female with scattered dark red papules and nodules with intense pruritus involving the lower limbs, upper limbs and trunk, with pronounced lesions over dorsa of hands and shins. | • TCS/Systemic CS. • Antihistamines. |
Label dosing. | Within 15 days of treatment, lesions over the dorsa of hands began to flatten and itching was slightly relieved, with progressive amelioration of symptoms as therapy progressed. | None. |
EBP: Epidermolysis bullosa pruriginosa, TCS: Topical corticosteroid, CS: Corticosteroids
GD
GD is an inflammatory immune disorder presenting as flesh-colored to erythematous, edematous papules, and/or papulovesicles, commonly involving the trunk and proximal extremities of middle aged men.[136] Apart from its idiopathic occurrence, GD has been reported following conventional chemotherapy, ICI therapy, recombinant human IL-4 therapy, and ipilumab (anti CTLA-4 monoclonal antibody) therapy.[137-140] The pathophysiology of GD is yet to be elucidated, though some reports suggest an overexpression of IL-4 to play a role.[141] Furthermore, with increasing age, the Th1/Th2 system is skewed toward a Th2-inflammation profile, with enhanced non-specific B-cell activation and IgE antibody production.[142,143] Based on these postulations, dupilumab has been employed in the treatment of GD. At present, two individual reports and a case series of three patients have elaborated dupilumab’s contribution in GD.[144-146] In all, within 12-14 weeks of dupilumab treatment, complete regression of dermatologic signs and pruritus was observed, without the occurrence of any untoward event.
HHD
HHD is an autosomal dominant genodermatosis principally characterized by flaccid blisters and malodorous erosions involving intertriginous areas, often accompanied by chronic pruritus. Mutations in the ATP2C1 gene are responsible for defective keratinocyte cell-to-cell adhesion, leading to acantholysis.[147] Although not indicated for HHD, few reports have delineated dupilumab to be effective for the same, details of which are outlined in Table 2.[148-151] As the mechanism of dupilumab in HHD is unclear, it is proposed that blockade of IL-4 and IL-13 may interfere with the agonistic effects of the cytokine CCL26 on CCR3, thereby preventing activation and chemotaxis of eosinophils, basophils, and Th2 lymphocytes, with subsequent inhibition of intracellular calcium release.[152] Right now, the optimal dose and frequency of dupilumab in HHD is not known, and some patients may benefit with more frequent injections.
| S. No. | Authors/Year | Study type | Patient details | Prior failed therapies | Dupilumab therapy | Remarks | Adverse effects |
|---|---|---|---|---|---|---|---|
| 1. | Alzahrani et al.,[148]/2021 | Case series of 3 patients. | • 2 females in their 50s. • 1 male in his 70s. |
• Isotretinoin. • Acitretin. • Etanercept. • Prednisolone. • Oral antibiotics. • Anti-histaminics. • Naltrexone. • Cyclosporine. • Local botulinum toxin. |
Label dosing. | Significant improvement was seen after an average of 2 months and sustained for17–25 months. | None. |
| 2. | Alamon-Reig et al.,[149]/2022 | Case series of 3 patients. | • 2 females (56 years/ 59 years). • 1 male (52 years). |
• Anti-histaminics. • Acitretin. • Systemic steroids. • Methotrexate. • Cyclosporine. • Hydroxychloroquine. • Naltrexone. • Apremilast. • Fluconazole. • Tetracycline. • Dapsone. • Oxybutynin. • Mycophenolate mofetil. • Local laser ablation. |
Label dosing. | • 2 patients improved with treatment. • One patient did not show any improvement. |
None. |
| 3. | Licata et al.,[150]/2022 | Case report. | 22-year-old female. | Cyclosporine. | Label dosing. | Resolution of lesions after 4 months of treatment. | None. |
| 4. | Brito Caldeira et al.,[151]/2023 |
Case report. | 57-year-old lady. | Low dose naltrexone. | Label dosing. | • Pruritus and sleep quality improved drastically after the first dose. • Significant healing of skin lesions was noticeable after 12 weeks. |
None. |
PV
PV is an intraepidermal immunobullous disorder targeting desmosomal adhesion proteins, desmoglein (Dsg)1, and Dsg 3.[153] Although systemic glucocorticoids remain the first-line therapy for PV, requirement for high dosage and long-term usage often herald untoward events. Moreover, other immunosuppressive drugs such as rituximab, azathioprine, MMF, cyclophosphamide, and methotrexate are not viable options in patients with an underlying infection.
At present, the utility of dupilumab in PV has been exemplified in two case reports.[154,155] In the first report (35-year-old male), the patient had PV along with pulmonary Kochs and was on antitubercular therapy. As an underlying sepsis was suspected, the dose of methylprednisolone (40 mg/day) could not be increased and dupilumab (label dosing) was added to the therapeutic regimen, along with antibiotics and low-dose IVIG (15 g for 4 days). The above combination resulted in regression of lesions with the pemphigus disease area index decreasing from 97 to 42 after 45 days of treatment, along with reduction of blood IgE levels and eosinophil count.[154] The second report delineated dupilumab (label dosing) monotherapy to be effective in PV following failed treatment with systemic steroids. Within 4 weeks, marked improvement was noticed, but as fledgeling oral lesions continued erupting within days before the next dose, weekly dupilumab (300 mg) injections were given that resulted in healing of all lesions by 6 weeks.[155]
In acute PV, higher concentrations of serum IgE and IgG4 antibodies are seen along with IgE epidermal deposits. As Th2 cells assist formation of IgG1 and IgG4 antibodies against Dsgs, dupilumab’s role in PV is postulated. By antagonizing IL-4, dupilumab affects IgE-related signaling through IL-4 on Th2 cells and ameliorates the pathogenic cascade.[156,157] RCTs with dupilumab in PV become essential to strengthen its utility for this indication.
CONCLUSION
Although primarily used for AD, dupilumab has been found to be useful in a number of off-label dermatologic indications. Besides, with a favorable safety profile, and an established and efficient clinical use for a broad range of populations, dupilumab holds promise as a newer treatment option for a plethora of dermatologic conditions. With numerous clinical trials ongoing, we expect dupilumab’s usage in dermatology to expand in the near future.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Dupilumab: A review of its use in the treatment of atopic dermatitis. J Am Acad Dermatol. 2018;78:S28-36.
- [CrossRef] [PubMed] [Google Scholar]
- Dupixent(dupilumab) approved by FDA as the first and only treatment indicated for prurigo nodularis. Available from: http://www.sanofi.com/en/media-room/press-releases/2022/2022-09-28-22-05-26-2524764 [Last accessed on 2024 Sep 01]
- [Google Scholar]
- Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2016;375:2335-48.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy and safety of multiple dupilumab dose regimens after initial successful treatment in patients with atopic dermatitis: A randomized clinical trial. JAMA Dermatol. 2020;156:131-43.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy and safety of dupilumab for the treatment of moderate-to-severe atopic dermatitis in adults: A pooled analysis of two phase 2 clinical trials. J Am Assoc Nurse Pract. 2018;30:529-41.
- [CrossRef] [PubMed] [Google Scholar]
- Dupilumab treatment in adults with moderate-to-severe atopic dermatitis. N Engl J Med. 2014;371:130-9.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy and safety of dupilumab in adults with moderate-to-severe atopic dermatitis inadequately controlled by topical treatments: A randomised, placebo-controlled, dose-ranging phase 2b trial. Lancet. 2016;387:40-52.
- [CrossRef] [PubMed] [Google Scholar]
- Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): A 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial. Lancet. 2017;389:2287-303.
- [CrossRef] [PubMed] [Google Scholar]
- Dupilumab improves the molecular signature in skin of patients with moderate-to-severe atopic dermatitis. J Allergy Clin Immunol. 2014;134:1293-300.
- [CrossRef] [PubMed] [Google Scholar]
- Thymus and activation-regulated chemokine in atopic dermatitis: Serum thymus and activation-regulated chemokine level is closely related with disease activity. J Allergy Clin Immunol. 2001;107:535-41.
- [CrossRef] [PubMed] [Google Scholar]
- Chronic prurigo of nodular type: A review. Acta Derm Venereol. 2018;98:173-9.
- [CrossRef] [PubMed] [Google Scholar]
- Nuclear localization of activated STAT6 and STAT3 in epidermis of prurigo nodularis. Br J Dermatol. 2011;165:990-6.
- [CrossRef] [PubMed] [Google Scholar]
- Sensory neurons co-opt classical immune signaling pathways to mediate chronic itch. Cell. 2017;171:217-28.e13.
- [CrossRef] [PubMed] [Google Scholar]
- Interactions of the immune and sensory nervous systems in atopy. FEBS J. 2018;285:3138-51.
- [CrossRef] [PubMed] [Google Scholar]
- Effectiveness and safety of dupilumab for the treatment of prurigo nodularis in a French multicenter adult cohort of 16 patients. J Eur Acad Dermatol Venereol. 2020;34:e74-6.
- [CrossRef] [PubMed] [Google Scholar]
- Dupilumab improves clinical manifestations, symptoms, and quality of life in adult patients with chronic nodular prurigo. J Am Acad Dermatol. 2020;83:39-45.
- [CrossRef] [PubMed] [Google Scholar]
- Dupilumab and prurigo nodularis-like phenotype in atopic dermatitis: Our experience of efficacy. J Dermatolog Treat. 2021;32:453-4.
- [CrossRef] [PubMed] [Google Scholar]
- Our experience with prurigo nodularis treated with dupilumab. J Eur Acad Dermatol Venereol. 2021;35:e285-7.
- [CrossRef] [PubMed] [Google Scholar]
- Effectiveness of dupilumab for the treatment of generalized prurigo nodularis phenotype of adult atopic dermatitis. Dermatitis. 2020;31:81-4.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy of dupilumab on different phenotypes of atopic dermatitis: One-year experience of 221 patients. J Clin Med. 2020;9:2684.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment of recalcitrant prurigo nodularis with dupilumab. SKIN J Cutan Med. 2020;4:279-83.
- [CrossRef] [Google Scholar]
- Dupilumab treatment for generalized prurigo nodularis. JAMA Dermatol. 2019;155:118-20.
- [CrossRef] [PubMed] [Google Scholar]
- Dupilumab for prurigo nodularis: Case series and review of the literature. Dermatol Ther. 2020;33:e13222.
- [CrossRef] [Google Scholar]
- Dupilumab in treatment of chronic prurigo: A case series and literature review. Acta Derm Venereol. 2019;99:905-6.
- [CrossRef] [PubMed] [Google Scholar]
- Dramatic improvement of generalized prurigo nodularis with dupilumab. J Eur Acad Dermatol Venereol. 2019;33:e303-4.
- [CrossRef] [PubMed] [Google Scholar]
- Effectiveness of dupilumab for chronic prurigo in elderly patients with atopic dermatitis. An Bras Dermatol. 2023;98:86-9.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy of dupilumab in prurigo nodularis in elderly patient. Dermatol Ther. 2020;33:e13201.
- [CrossRef] [Google Scholar]
- Safety and effectiveness of dupilumab in prurigo nodularis. J Investig Allergol Clin Immunol. 2021;31:162-3.
- [CrossRef] [PubMed] [Google Scholar]
- Simultaneous treatment of Samter triad and prurigo nodularis with dupilumab. JAAD Case Rep. 2021;18:20-2.
- [CrossRef] [PubMed] [Google Scholar]
- Dupilumab as a useful treatment option for prurigo nodularis in an elderly patient with atopic diathesis. Int J Dermatol. 2020;59:e358-61.
- [CrossRef] [Google Scholar]
- Dupilumab treatment for prurigo nodularis and pruritis. J Drugs Dermatol. 2019;18:940-2.
- [Google Scholar]
- Dupilumab for pediatric prurigo nodularis: A case report. Pediatr Dermatol. 2021;38:334-5.
- [CrossRef] [PubMed] [Google Scholar]
- Effectiveness of dupilumab for an elderly patient with prurigo nodularis who was refractory and contradicted to traditional therapy. J Asthma Allergy. 2021;14:175-8.
- [CrossRef] [PubMed] [Google Scholar]
- Resolution of treatment-refractory prurigo nodularis with dupilumab: A Case Series. Cureus. 2020;12:e8737.
- [CrossRef] [PubMed] [Google Scholar]
- Dupilumab for treatment-refractory prurigo nodularis. J Dtsch Dermatol Ges. 2020;18:618-24.
- [CrossRef] [Google Scholar]
- Dupilumab in prurigo nodularis: a systematic review of current evidence and analysis of predictive factors to response. J Dermatolog Treat. 2022;33:1547-53.
- [CrossRef] [PubMed] [Google Scholar]
- Hand eczema: Causes, course, and prognosis I. Contact Dermatitis. 2008;58:330-4.
- [CrossRef] [Google Scholar]
- Vesicular hand eczema transcriptome analysis provides insights into its pathophysiology. Exp Dermatol. 2021;30:1775-86.
- [CrossRef] [PubMed] [Google Scholar]
- Successful treatment of recalcitrant dyshidrotic eczema with dupilumab in a child. J Dtsch Dermatol Ges. 2019;17:1165-7.
- [CrossRef] [Google Scholar]
- Occupational chronic hand dermatitis in hospital environment successfully treated with dupilumab: A case report. Iran J Allergy Asthma Immunol. 2022;21:484-7.
- [CrossRef] [PubMed] [Google Scholar]
- Dupilumab for occupational irritant hand dermatitis in a nonatopic individual: A case report. JAAD Case Rep. 2020;6:296-8.
- [CrossRef] [PubMed] [Google Scholar]
- A case of complete resolution of severe plantar dyshidrotic eczema with dupilumab. J Drugs Dermatol. 2019;18:211-2.
- [Google Scholar]
- Dupilumab treatment of very severe refractory atopic hand eczema. JAMA Dermatol. 2018;154:969-70.
- [CrossRef] [PubMed] [Google Scholar]
- Severe treatment-resistant acute and recurrent vesicular chronic hand eczema successfully treated with dupilumab. Contact Dermatitis. 2020;83:37-8.
- [CrossRef] [PubMed] [Google Scholar]
- Two cases of recalcitrant dyshidrotic eczema treated with dupilumab. J Drugs Dermatol. 2021;20:558-9.
- [Google Scholar]
- Dupilumab in the treatment of dyshidrosis: A report of two cases. J Drugs Dermatol. 2018;17:355-6.
- [Google Scholar]
- Three cases of nonatopic hyperkeratotic hand eczema treated with dupilumab. Contact Dermatitis. 2021;84:124-7.
- [CrossRef] [PubMed] [Google Scholar]
- Dupilumab for the treatment of dyshidrotic eczema in 15 consecutive patients. J Am Acad Dermatol. 2020;82:1251-2.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of dupilumab on hand eczema in patients with atopic dermatitis: An observational study. J Dermatol. 2019;46:680-5.
- [CrossRef] [PubMed] [Google Scholar]
- The long-term effect of dupilumab on chronic hand eczema in patients with moderate to severe atopic dermatitis-52 week results from the Dutch BioDay Registry. Contact Dermatitis. 2022;87:185-91.
- [CrossRef] [PubMed] [Google Scholar]
- A retrospective review of dupilumab for hand dermatitis. Dermatology. 2019;235:187-8.
- [CrossRef] [PubMed] [Google Scholar]
- Dupilumab: A review of present indications and off-label uses. J Investig Allergol Clin Immunol. 2022;32:97-115.
- [CrossRef] [PubMed] [Google Scholar]
- Repeated elicitation of contact hypersensitivity induces a shift in cutaneous cytokine milieu from a T helper cell type 1 to a T helper cell type 2 profile. J Immunol. 1997;159:2484-91.
- [CrossRef] [PubMed] [Google Scholar]
- Impaired contact hypersensitivity to trinitrochlorobenzene in interleukin-4-deficient mice. Immunology. 1999;98:71-9.
- [CrossRef] [PubMed] [Google Scholar]
- Interleukin-4 and the interleukin-4 receptor in allergic contact dermatitis. Contact Dermatitis. 1998;38:36-9.
- [CrossRef] [PubMed] [Google Scholar]
- Enhanced expression levels of IL-31 correlate with IL-4 and IL-13 in atopic and allergic contact dermatitis. J Allergy Clin Immunol. 2006;118:930-7.
- [CrossRef] [PubMed] [Google Scholar]
- A retrospective review of dupilumab for atopic dermatitis patients with allergic contact dermatitis. J Am Acad Dermatol. 2019;80:1166-7.
- [CrossRef] [PubMed] [Google Scholar]
- Effective use of dupilumab in managing systemic allergic contact dermatitis. Dermatitis. 2018;29:282-4.
- [CrossRef] [PubMed] [Google Scholar]
- A case series of dupilumab-treated allergic contact dermatitis patients. Dermatol Ther. 2018;31:e12701.
- [CrossRef] [PubMed] [Google Scholar]
- Dupilumab treatment in two patients with severe allergic contact dermatitis caused by sesquiterpene lactones. Contact Dermatitis. 2020;83:137-9.
- [CrossRef] [PubMed] [Google Scholar]
- Occupational chronic contact dermatitis successfully treated with dupilumab: A case series. Dermatology. 2022;238:1073-5.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of dupilumab on allergic contact dermatitis and patch testing. J Am Acad Dermatol. 2021;84:1772-6.
- [CrossRef] [PubMed] [Google Scholar]
- Repeat patch testing in a patient with allergic contact dermatitis improved on dupilumab. JAAD Case Rep. 2019;5:336-8.
- [CrossRef] [PubMed] [Google Scholar]
- Dupilumab use in allergic contact dermatitis. J Am Acad Dermatol. 2019;80:280-1.e1.
- [CrossRef] [PubMed] [Google Scholar]
- Dupilumab for systemic allergy syndrome with dermatitis. Dermatitis. 2019;30:164-7.
- [CrossRef] [PubMed] [Google Scholar]
- Variable impact of dupilumab on patch testing results and allergic contact dermatitis in adults with atopic dermatitis. J Am Acad Dermatol. 2019;81:157-62.
- [CrossRef] [PubMed] [Google Scholar]
- Recall dermatitis at patch test sites in an atopic dermatitis patient treated with dupilumab. Contact Dermatitis. 2019;80:69-70.
- [CrossRef] [PubMed] [Google Scholar]
- Autoimmune diseases in adults with atopic dermatitis. J Am Acad Dermatol. 2017;76:274-80.e1.
- [CrossRef] [PubMed] [Google Scholar]
- The changing landscape of alopecia areata: The therapeutic paradigm. Adv Ther. 2017;34:1594-609.
- [CrossRef] [PubMed] [Google Scholar]
- Long-term efficacy of dupilumab in alopecia areata. Am J Case Rep. 2022;23:e936488.
- [CrossRef] [Google Scholar]
- Alopecia areata: An updated review for 2023. J Cutan Med Surg. 2023;27:241-59.
- [CrossRef] [PubMed] [Google Scholar]
- Two-sided influence of dupilumab on alopecia areata co-existing with severe atopic dermatitis: A case series and literature review. J Cut Immunol Allergy. 2023;6:13-7.
- [CrossRef] [Google Scholar]
- Drug-induced alopecia after dupilumab therapy. JAAD Case Rep. 2019;5:54-6.
- [CrossRef] [PubMed] [Google Scholar]
- Development of alopecia in patients treated with dupilumab. Dermatol Ther. 2019;32:e12869.
- [CrossRef] [Google Scholar]
- Alopecia areata development in atopic dermatitis patients treated with dupilumab. J Eur Acad Dermatol Venereol. 2020;34:e612-3.
- [CrossRef] [Google Scholar]
- Improvement of atopic dermatitis and alopecia universalis with dupilumab. Dermatol Reports. 2022;14:9359.
- [CrossRef] [PubMed] [Google Scholar]
- Onset of Schamberg disease and resolution of alopecia areata during treatment of atopic dermatitis with dupilumab. J Investig Allergol Clin Immunol. 2021;31:65-6.
- [CrossRef] [PubMed] [Google Scholar]
- Rapid hair regrowth induced by dupilumab in a patient affected by alopecia totalis of 28 years' duration: Clinical and dermoscopic features. Dermatol Ther. 2020;33:e13582.
- [CrossRef] [Google Scholar]
- Dupilumab-induced psoriasis in a patient with atopic dermatitis and alopecia totalis: A case report and literature review. Dermatol Ther. 2022;35:e15255.
- [CrossRef] [PubMed] [Google Scholar]
- Dupilumab for the treatment of alopecia areata in children with atopic dermatitis. JAAD Case Rep. 2021;16:82-5.
- [CrossRef] [PubMed] [Google Scholar]
- Simultaneous effectiveness of dupilumab in atopic dermatitis and alopecia areata in two patients. J Dtsch Dermatol Ges. 2019;17:1278-80.
- [CrossRef] [Google Scholar]
- The effectiveness of dupilumab in patients with alopecia areata who have atopic dermatitis: A case series of seven patients. Br J Dermatol. 2020;183:396-7.
- [CrossRef] [PubMed] [Google Scholar]
- Case of alopecia areata during dupilumab treatment for atopic dermatitis. J Dermatol. 2019;46:e332-3.
- [CrossRef] [Google Scholar]
- Massive acute alopecia of the scalp in a patient treated with dupilumab. Acta Derm Venereol. 2020;100:adv00191.
- [CrossRef] [PubMed] [Google Scholar]
- Alopecia areata after dupilumab for atopic dermatitis. JAAD Case Rep. 2018;4:143-4.
- [CrossRef] [PubMed] [Google Scholar]
- Alopecia areata in severe atopic dermatitis treated with dupilumab. J Investig Allergol Clin Immunol. 2018;28:420-1.
- [CrossRef] [PubMed] [Google Scholar]
- Phase 2a randomized clinical trial of dupilumab (anti-IL-4Rα) for alopecia areata patients. Allergy. 2022;77:897-906.
- [CrossRef] [PubMed] [Google Scholar]
- The international EAACI/GA2LEN/EuroGuiDerm/APAAACI guideline for the definition, classification, diagnosis, and management of urticaria. Allergy. 2022;77:734-66.
- [CrossRef] [PubMed] [Google Scholar]
- Severe chronic spontaneous urticaria in children-treatment options according to the guidelines and beyond-a 10 years review. J Dermatolog Treat. 2022;33:1119-22.
- [CrossRef] [PubMed] [Google Scholar]
- Rapid disappearance of both severe atopic dermatitis and cold urticaria following dupilumab treatment. Clin Exp Dermatol. 2020;45:345-6.
- [CrossRef] [PubMed] [Google Scholar]
- Dupilumab as a rescue therapy for a chronic urticaria patient who showed secondary failure to omalizumab. Kaohsiung J Med Sci. 2022;38:610-1.
- [CrossRef] [PubMed] [Google Scholar]
- Dupilumab as a novel therapy to treat adrenergic urticaria. Ann Allergy Asthma Immunol. 2021;126:205-6.
- [CrossRef] [PubMed] [Google Scholar]
- Complete response to dupilumab in a patient with chronic spontaneous urticaria who did not tolerate omalizumab. JAAD Case Rep. 2022;32:109-12.
- [CrossRef] [PubMed] [Google Scholar]
- Cholinergic urticaria, an effective and safe “off label” use of dupilumab: A case report with literature review. Clin Cosmet Investig Dermatol. 2022;15:253-60.
- [CrossRef] [PubMed] [Google Scholar]
- Case report: Severe chronic spontaneous urticaria successfully treated with omalizumab and dupilumab. Allergol Select. 2023;7:17-9.
- [CrossRef] [PubMed] [Google Scholar]
- Concurrent use of omalizumab and dupilumab in a 47-year-old woman with chronic spontaneous urticaria and atopic dermatitis. Int J Dermatol. 2022;61:e173-4.
- [CrossRef] [PubMed] [Google Scholar]
- Long-term follow-up of patients treated with dupilumab for chronic spontaneous urticaria: A case report. SAGE Open Med Case Rep. 2022;10:2050313X221117702.
- [CrossRef] [PubMed] [Google Scholar]
- Dupilumab as a novel therapy for difficult to treat chronic spontaneous urticaria. J Allergy Clin Immunol Pract. 2019;7:1659-61.e1.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacity of dupilumab in severe idiopathic cold urticaria: A case report. J Dermatolog Treat. 2023;34:2182620.
- [CrossRef] [PubMed] [Google Scholar]
- Mast cell production and response to IL-4 and IL-13. Cytokine. 2015;75:57-61.
- [CrossRef] [PubMed] [Google Scholar]
- IL-4 and human skin mast cells revisited: Reinforcement of a pro-allergic phenotype upon prolonged exposure. Arch Dermatol Res. 2016;308:665-70.
- [CrossRef] [PubMed] [Google Scholar]
- Pruritus as a distinctive feature of type 2 inflammation. Vaccines (Basel). 2021;9:303.
- [CrossRef] [PubMed] [Google Scholar]
- Dupilumab in patients with chronic spontaneous urticaria (LIBERTY-CSU CUPID): Two randomized, double-blind, placebo-controlled, phase 3 trials. J Allergy Clin Immunol. 2024;154:184-94.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy and safety of dupilumab in moderate-to-severe bullous pemphigoid. Front Immunol. 2021;12:738907.
- [CrossRef] [PubMed] [Google Scholar]
- Review of autoimmune blistering diseases: The Pemphigoid diseases. J Eur Acad Dermatol Venereol. 2019;33:1685-94.
- [CrossRef] [PubMed] [Google Scholar]
- Skin-homing interleukin-4 and-13-producing cells contribute to bullous pemphigoid: Remission of disease is associated with increased frequency of interleukin-10-producing cells. J Invest Dermatol. 2001;117:1097-102.
- [CrossRef] [PubMed] [Google Scholar]
- Human eosinophils express the high affinity IgE receptor, FcεRI, in bullous pemphigoid. PLoS One. 2014;9:e107725.
- [CrossRef] [PubMed] [Google Scholar]
- Up-regulation of CCL11 and CCL26 is associated with activated eosinophils in bullous pemphigoid. Clin Exp Immunol. 2011;166:145-53.
- [CrossRef] [PubMed] [Google Scholar]
- Pathophysiologic mechanisms of itch in bullous pemphigoid. J Am Acad Dermatol. 2020;83:53-62.
- [CrossRef] [PubMed] [Google Scholar]
- Dupilumab as a novel therapy for bullous pemphigoid: A multicenter case series. J Am Acad Dermatol. 2020;83:46-52.
- [CrossRef] [PubMed] [Google Scholar]
- Dupilumab as a novel therapy for bullous pemphigoid. Int J Dermatol. 2023;62:e263-6.
- [CrossRef] [Google Scholar]
- Dupilumab for the treatment of recalcitrant bullous pemphigoid. JAMA Dermatol. 2018;154:1225-6.
- [CrossRef] [PubMed] [Google Scholar]
- Dupilumab for bullous pemphigoid with intractable pruritus. Dermatol Online J. 2019;25:13030/qt25q9w6r9.
- [CrossRef] [PubMed] [Google Scholar]
- Recalcitrant bullous pemphigoid responsive to dupilumab in an adolescent patient. JAAD Case Rep. 2022;29:149-51.
- [CrossRef] [PubMed] [Google Scholar]
- Case report: Combination of omalizumab and dupilumab for recalcitrant bullous pemphigoid. Front Immunol. 2020;11:611549.
- [CrossRef] [PubMed] [Google Scholar]
- Successful treatment of bullous pemphigoid with dupilumab: A case and brief review of the literature. Dermatol Online J. 2021;27:13030/qt0dv3f9h6.
- [CrossRef] [PubMed] [Google Scholar]
- Dupilumab combined with low-dose systemic steroid therapy improves efficacy and safety for bullous pemphigoid. Dermatol Ther. 2022;35:e15648.
- [CrossRef] [Google Scholar]
- Dupilumab for the treatment of nivolumab-induced bullous pemphigoid: A case report and review of the literature. Dermatol Online J. 2021;27:10.5070/D327955136.
- [CrossRef] [PubMed] [Google Scholar]
- Dupilumab for the treatment of pembrolizumab-induced bullous pemphigoid: A case report. Dermatol Ther. 2022;35:e15623.
- [CrossRef] [PubMed] [Google Scholar]
- A successful case of vesicular pemphigoid concurrent with pulmonary tuberculosis with dupilumab. Dermatol Ther. 2022;35:e15330.
- [CrossRef] [PubMed] [Google Scholar]
- Nonbullous erythrodermic pemphigoid with florid lymphadenopathy, response to dupilumab. JAAD Case Rep. 2021;17:58-60.
- [CrossRef] [PubMed] [Google Scholar]
- Severe pemphigoid nodularis successfully treated with dupilumab. Dermatol Ther. 2022;35:e15727.
- [CrossRef] [PubMed] [Google Scholar]
- Fetal morbidity in herpes gestationis. Arch Dermatol. 1995;131:1209-10.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment of pemphigoid gestationis with dupilumab. Clin Exp Dermatol. 2021;46:1578-9.
- [CrossRef] [PubMed] [Google Scholar]
- Benign pemphigold: A report of seven cases with chronic, scarring, herpetiform plaques about the head and neck. AMA Arch Derm. 1957;75:489-501.
- [CrossRef] [PubMed] [Google Scholar]
- Successful treatment of Brunsting-Perry pemphigoid with dupilumab. JAAD Case Rep. 2021;10:107-9.
- [CrossRef] [PubMed] [Google Scholar]
- Successful treatment of brunsting-perry cicatricial pemphigoid with dupilumab. J Drugs Dermatol. 2021;20:1113-5.
- [Google Scholar]
- Allelic heterogeneity of dominant and recessive COL7A1 mutations underlying epidermolysis bullosa pruriginosa. J Invest Dermatol. 1999;112:984-7.
- [CrossRef] [PubMed] [Google Scholar]
- Amelioration of dystrophic epidermolysis bullosa pruriginosa symptoms with dupilumab: A case report. Dermatol Ther. 2021;34:e15130.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment of epidermolysis bullosa pruriginosa-associated pruritus with dupilumab. Br J Dermatol. 2020;182:1495-7.
- [CrossRef] [PubMed] [Google Scholar]
- Epidermolysis bullosa pruriginosa responding to dupilumab. JAAD Case Rep. 2021;16:69-71.
- [CrossRef] [PubMed] [Google Scholar]
- Epidermolysis bullosa pruriginosa treated with dupilumab. Pediatr Dermatol. 2021;38:526-7.
- [CrossRef] [PubMed] [Google Scholar]
- Grover disease: Review of subtypes with a focus on management options. Int J Dermatol. 2020;59:543-50.
- [CrossRef] [PubMed] [Google Scholar]
- Grover disease associated with chemotherapy: Review of potential pathophysiology, current treatments, and future directions. J Drugs Dermatol. 2020;19:1056-64.
- [CrossRef] [PubMed] [Google Scholar]
- Suprabasal acantholytic dermatologic toxicities associated checkpoint inhibitor therapy: A spectrum of immune reactions from paraneoplastic pemphigus-like to Grover-like lesions. J Cutan Pathol. 2018;45:764-73.
- [CrossRef] [PubMed] [Google Scholar]
- Transient acantholytic dermatosis induced by recombinant human interleukin 4. J Am Acad Dermatol. 1993;29:206-9.
- [CrossRef] [PubMed] [Google Scholar]
- First report of ipilimumab-induced Grover disease. Br J Dermatol. 2014;171:1236-7.
- [CrossRef] [PubMed] [Google Scholar]
- IL-4 regulates skin homeostasis and the predisposition toward allergic skin inflammation. J Immunol. 2010;184:3186-90.
- [CrossRef] [PubMed] [Google Scholar]
- Age-dependent modifications of Type 1 and Type 2 cytokines within virgin and memory CD4+ T cells in humans. Mech Ageing Dev. 2006;127:560-6.
- [CrossRef] [PubMed] [Google Scholar]
- A case of Grover disease treated with Dupilumab: Just serendipity or a future perspective? Dermatol Ther. 2022;35:e15429.
- [CrossRef] [Google Scholar]
- Can't handle the itch? Refractory immunotherapy-related transient acantholytic dermatosis: Prompt resolution with dupilumab. JAAD Case Rep. 2022;22:31-3.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment of grover disease with dupilumab. JAMA Dermatol. 2021;157:353-6.
- [CrossRef] [PubMed] [Google Scholar]
- Mutations in ATP2C1, encoding a calcium pump, cause Hailey-Hailey disease. Nat Genet. 2000;24:61-5.
- [CrossRef] [PubMed] [Google Scholar]
- Hailey-Hailey disease treated with dupilumab: A case series. Br J Dermatol. 2021;185:680-2.
- [CrossRef] [PubMed] [Google Scholar]
- Dupilumab in HaileyHailey disease: A case series. J Eur Acad Dermatol Venereol. 2022;36:e776-9.
- [CrossRef] [Google Scholar]
- A case of Hailey-Hailey disease successfully treated with dupilumab. Int J Dermatol. 2022;61:1427-8.
- [CrossRef] [PubMed] [Google Scholar]
- Successful treatment of Hailey-Hailey disease with dupilumab. Actas Dermosifiliogr. 2023;114:914-5.
- [CrossRef] [Google Scholar]
- Eotaxin-3/CCL26 is a natural antagonist for CC chemokine receptors 1 and 5. A human chemokine with a regulatory role. J Biol Chem. 2004;279:23357-63.
- [CrossRef] [PubMed] [Google Scholar]
- A novel combined use of dupilumab for treatment of aggressive refractory pemphigus vulgaris complicated with pulmonary tuberculosis: A case report and the RNA-seq analysis. Front Immunol. 2022;13:825796.
- [CrossRef] [PubMed] [Google Scholar]
- Dupilumab monotherapy suppresses recalcitrant pemphigus vulgaris. JAAD Case Rep. 2023;31:16-8.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical activity of pemphigus vulgaris relates to IgE autoantibodies against desmoglein 3. Clin Immunol. 2010;134:320-30.
- [CrossRef] [PubMed] [Google Scholar]
- Pathogenic IgG antibodies against desmoglein 3 in pemphigus vulgaris are regulated by HLA-DRB1*04:02-restricted T cells. J Immunol. 2014;193:4391-9.
- [CrossRef] [PubMed] [Google Scholar]







