Translate this page into:
Necrolytic acral erythema as a manifestation of Crohn’s disease and celiac disease - A report of two cases
*Corresponding author: Anza Khader, Department of Dermatology, Government Medical College, Kozhikode, Kerala, India. anzashaan@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Khader A, Sreekanth S, Manakkattu SP, John N. Necrolytic acral erythema as a manifestation of Crohn’s disease and celiac disease - A report of two cases. J Skin Sex Transm Dis 2020;2(2):130-3.
Abstract
Necrolytic acral erythema (NAE) is considered as a diagnostic marker of hepatitis C infection. Here, we report two cases of NAE in Crohn’s disease and celiac disease. Both the patients were seronegative for hepatitis C virus. The first case presented with recurrent diarrhea, weight loss, bullae on hyperpigmented plaque over extremities and gastrointestinal endoscopy and biopsy consistent with Crohn’s disease. The second case presented with recurrent diarrhea, vomiting, vesiculopustules and scaly plaques over extremities and duodenal biopsy diagnostic of celiac disease. NAE presents as papules and plaques with bullae over extremities, but tend to spare palms and soles. Our first patient had lesions on sole and the second patient had pustular lesions. To the best of our knowledge, there are no available reports of association of NAE with Crohn’s disease or celiac disease.
Keywords
Necrolytic acral erythema
Crohn’s disease
Celiac disease
Zinc
INTRODUCTION
Necrolytic acral erythema (NAE) was first described by El Darouti and El Ela in 1996 in seven patients with hepatitis C infection and is considered as its diagnostic marker.[1] However, there are many contrary reports of NAE in hepatitis C virus seronegative.[2-4] Low zinc levels in NAE and the therapeutic response to zinc suggest a physiological basis for classifying NAE among the necrolytic erythema linked to nutrient deficiencies.[5] Some consider it as a variant of necrolytic migratory erythema (NME) and an alternative term of acral NME has been proposed.[6] Unlike NME, NAE is not reported in literature in association with gastrointestinal diseases. Here, we report two cases of NAE as a cutaneous marker of underlying Crohn’s disease and celiac disease, both of them seronegative for hepatitis C virus.
CASE REPORT
Case 1
A 31-year-old male presented with recurrent episodes of fluid-filled lesions over hands and feet of 3 months duration. He had multiple episodes of diarrhea and weight loss for the past 1 year. He had no addictions and no history of sexual exposures. General examination revealed marfanoid habitus, facial puffiness, diffuse thyroid enlargement, clubbing and bilateral pitting pedal edema. Dermatological examination showed multiple bullae on well-defined hyperpigmented plaques with a dusky hue over the dorsum of toes and feet extending to instep area and over interdigital spaces of fingers and dorsa of hands [Figure 1a and b]. Hair and nails were normal. There were no mucosal erosions. Routine blood investigations revealed normal blood counts, low serum albumin of 1.7 g/dl and hypocholesterolemia. Thyroid function test showed low serum T3 and T4 and high thyroid stimulating hormone. Serological tests for antibodies to human immunodeficiency virus and hepatitis A, B, and C were negative. Stool examination showed the presence of fat globules. Skin biopsy showed hyperkeratosis, parakeratosis, acanthosis, spongiosis, suprabasal split, and perivascular dermal infiltrate [Figure 2]. Serum zinc levels were found to be as low as 50 μg/dl (normal: 84–159 μg/dl). Contrast-enhanced computed tomography abdomen showed long segment thickening involving ileum with multiple mesenteric lymph nodes. Upper gastrointestinal endoscopy and biopsy revealed granulomatous infiltrate in the lamina propria of ileum [Figure 3]. The patient was diagnosed as NAE with Crohn’s disease. The skin lesions subsided within 2 weeks after starting zinc supplementation at a dose of 220 mg twice daily [Figure 4a and b]. The patient was started on azathioprine 100 mg as a treatment for Crohn’s disease.
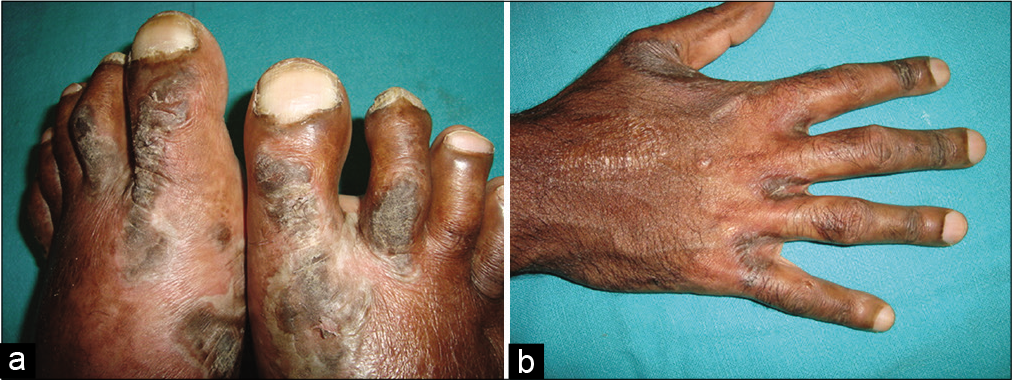
- (a) Bullae on well-defined hyperpigmented plaques over the dorsum of toes and feet. (b) Collapsed bullae over interdigital areas of fingers.

- Hyperkeratosis, parakeratosis, acanthosis, spongiosis, and suprabasal split (H&E, ×200).

- Granulomatous infiltrate in lamina propria of ileum (H&E, ×200).

- (a) Subsiding lesions over dorsa of the left foot after 2 weeks of treatment. (b) Lesions over interdigital area subsided completely after 2 weeks of treatment.
Case 2
A 28-year-old male presented with pruritic oozy lesions over fingers, interdigital areas, and ankles of 4 months duration. He had multiple episodes of vomiting and watery diarrhea following intake of specific food items. He sustained a loss of 15 kg during the past 4 months.
General examination revealed pallor, clubbing, glossitis, and graying of hair. Dermatological examination showed scaly plaques with vesicles and pustules over interdigital area and dorsa of fingers, hyperpigmented plaques with pustules over lower legs [Figure 5]. There were no mucosal erosions. Routine blood investigations revealed reduced hemoglobin of 10 g/dl, macrocytes in peripheral smear and raised ESR 75 mm in the 1st hour. Serum iron was normal. Serum zinc was low at 52 μg/dl and serum Vitamin B12 was 123.5 pg/ ml (normal: 200–600 pg/ml). Serological tests for antibodies to human immunodeficiency virus and hepatitis A, B, and C were negative. Skin biopsy showed parakeratosis, subcorneal pustules, irregular acanthosis, suprabasal split, and perivascular lymphohistiocytic infiltrate. Ultrasonography and computerized tomography of abdomen revealed multiple mesenteric lymph nodes. Upper gastrointestinal endoscopy and duodenal biopsy showed focal villi atrophy, focal crypt hyperplasia, and focal increase in intraepithelial lymphocytes consistent with celiac disease with marsh score 3b [Figures 6a and b]. Anti-endomysial antibodies and antitransglutaminase antibodies were negative. The patient was diagnosed as NAE with celiac disease and was advised rice-based gluten-free diet and oral zinc sulfate 220 mg twice daily. The skin lesions subsided within 1 month.

- Scaly plaques with vesicles and pustules over interdigital area of the right hand.

- (a) Intraepithelial lymphocytes in duodenal biopsy (H&E ×400). (b) Intraepithelial CD 3+ lymphocytes on immunohistochemistry (×200).
DISCUSSION
The exact pathogenesis of NAE is not known beyond doubt. Hepatocellular dysfunction resulting in hypoaminoacidemia and hyperglucagonemia has been proposed. Hypoaminoacidemia leads to loss of epidermal proteins and necrolysis. Hypoalbuminemia increases the levels of prostaglandins, which is thought to induce inflammatory process in NAE.[6] Albumin being the carrier of zinc, low albumin can reduce zinc levels.[7]
Both of our patients were seronegative for hepatitis A, B, and C. However, both of them had recurrent diarrhea, weight loss, and clubbing signifying an underlying systemic disease.
Hypoalbuminemia was noted in Case 1.
NAE clinically presents with bullous lesions in the acute stage, erythematous or lichenoid plaques with a dusky hue when fully evolved and with hyperpigmentation in the late stage.[8] Clinical presentation of HCV seronegative NAE includes itchy or non-itchy scaly erythematous papules or plaques and rarely vesicles and bulla. The most common sites of involvement are the dorsal aspects of the feet, over the Achilles tendons, malleoli, legs, and knees. Elbows, hands, buttocks, and genitalia are less frequently involved and palms, soles, face, and mucous membranes are often spared.[9] However, there are a few reports of palms and sole involvement and therefore palmar and plantar lesions do not exclude the diagnosis.[10] Case 1 presented with bullous lesions and hyperpigmented plaques with a dusky hue typically distributed on acral areas with extension to the sole and involvement of interdigital spaces of hand. Case 2 had scaly plaques with pustulation.
Histologically, NAE is characterized by acanthosis, epidermal spongiosis, and perivascular dermatitis in acute stage and psoriasiform hyperplasia, subcorneal pustules, and necrotic keratinocytes when fully evolved. Confluent necrosis of keratinocytes may lead to cleft formation. Our first patient had acanthosis, spongiosis, and perivascular infiltrate. Suprabasal cleft may be due to confluent necrosis of lower epidermal keratinocytes. The second case demonstrated psoriasiform hyperplasia and subcorneal pustules along with suprabasal split. Due to the absence of specific histological findings, diagnosis of NAE should be considered even in the absence of classical histology.[10]
The presence of fat globules in stool and decreased serum cholesterol in case 1 prompted us to evaluate the patient for any malabsorption. The CT finding and biopsy were consistent with Crohn’s disease. Zinc deficiency is known to be associated with Crohn’s disease.[11] The malabsorption due to Crohn’s disease might have resulted in steatorrhea, hypoalbuminemia, and reduced zinc level. Celiac disease is characterized by the inability to tolerate gliadin, the alcohol-soluble fraction of gluten which leads to damage of the intestinal mucosa with comorbid malabsorption.
Case 2 had glossitis, graying of hair and macrocytic anemia which are features of vitamin B12 deficiency, and had skin lesions of zinc deficiency. Zinc deficiency may result from a cumulative loss of insoluble zinc complexes with fat and phosphate or from impaired absorption.[12]
Zinc deficiency has been suggested as an etiologic factor for NAE, due to low levels of zinc in a subset of patients and the clinical response to zinc supplementation seen in many patients. However, the mechanisms of therapeutic response to zinc still remain to be elucidated.[5,10]
NAE needs to be considered as a separate entity not exclusively associated with HCV. Bullous lesions typical of HCV seropositive NAE were the presenting symptom in our first patient who was seronegative. The association of NAE with celiac disease and Crohn’s disease is not reported in literature. The clinicians should be aware of this rare entity of NAE which might be a pointer to the underlying gastrointestinal diseases.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
Dr. Sukumarakurup Sreekanth, Dr. Anza Khader, and Dr. Nimmi John are on the Editorial Board of the Journal.
References
- Necrolytic acral erythema: A cutaneous marker of viral Hepatitis C. Int J Dermatol. 1996;35:252-6.
- [CrossRef] [Google Scholar]
- Necrolytic acral erythema seronegative for Hepatitis C virus-two cases from India treated with oral zinc. Int J Dermatol. 2009;48:1096-9.
- [CrossRef] [PubMed] [Google Scholar]
- Necrolytic acral erythema without Hepatitis C infection. J Cutan Pathol. 2009;36:355-8.
- [CrossRef] [Google Scholar]
- Seronegative necrolytic acral erythema: A distinct clinical subset? Indian J Dermatol. 2010;55:259-61.
- [CrossRef] [Google Scholar]
- Zinc deficiency associated with necrolytic acral erythema. J Am Acad Dermatol. 2006;55:108-10.
- [CrossRef] [Google Scholar]
- Necrolytic acral erythema: A variant of necrolytic migratory erythema or a distinct entity? Int J Dermatol. 2005;44:916-21.
- [CrossRef] [Google Scholar]
- Necrolytic acral erythema: A patient from the United States successfully treated with oral zinc. Arch Dermatol. 2005;141:85-7.
- [CrossRef] [PubMed] [Google Scholar]
- Necrolytic acral erythema: A cutaneous sign of Hepatitis C virus infection. J Am Acad Dermatol. 2005;53:247-51.
- [CrossRef] [Google Scholar]
- Necrolytic acral erythema in the absence of Hepatitis C virus infection. Indian J Dermatol. 2016;61:96-8.
- [CrossRef] [Google Scholar]
- Lack of classic histology should not prevent diagnosis of necrolytic acral erythema. J Am Acad Dermatol. 2009;60:504-7.
- [CrossRef] [Google Scholar]
- Zinc supplementation to patients with celiac disease-is it required? J Trop Pediatr. 2010;56:391-7.
- [CrossRef] [Google Scholar]






