Translate this page into:
Papulopustular rash following Moderna COVID-19 (mRNA-1273) vaccine: A case report
*Corresponding author: David Pudukadan, Department of Dermatology, Jubilee Mission Medical College and Research Institute, Thrissur, Kerala, India. davidpudukadan@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Pudukadan D. Papulopustular rash following Moderna COVID-19 (mRNA-1273) vaccine: A case report. J Skin Sex Transm Dis 2022;4:110-4.
Abstract
Coronavirus disease-2019 (COVID-19) has affected countries around the world. The introduction of COVID-19 vaccines has proved the most effective arsenal in the fight against the disease. However, with the vaccination of billions of people, data on vaccine-induced adverse reactions are also emerging. We report a 32-year-old woman who manifested papulopustular rash 7 days after receiving Moderna COVID-19 (mRNA-1273) vaccine. The patient responded to a short course of systemic steroids and antihistamines. Awareness regarding the possible adverse events that can be anticipated after the COVID-19 vaccination may help the healthcare professionals to offer prompt and effective care to the affected.
Keywords
Coronavirus disease-2019
Papulopustular rash
Moderna COVID-19 (mRNA-1273) vaccine
Systemic steroids
COVID-19 vaccination
Adverse event
INTRODUCTION
Billions of people worldwide have received coronavirus disease-2019 (COVID-19) vaccines, and the available data support the efficacy of the vaccines against the disease.[1] As more and more people receive vaccines, adverse events to vaccines are also being reported. We report a patient who developed papulopustular rash following Moderna COVID-19 (mRNA-1273) vaccine.
CASE REPORT
A 32-year-old Indian woman with no history of allergic reactions to any food items or drugs presented with a 3-day history of pruritic, red, and raised lesions that appeared all over the body. She had no other symptoms. She had developed generalized itching 3 days back and was given one tablet of 4 mg chlorpheniramine maleate. She denied taking any medicines before the onset of the lesions, but had received the third (booster) dose of Moderna COVID-19 (mRNA-1273) vaccine 7 days before the onset of the rash. She did not develop any adverse events following the first two doses of the vaccine. She did not give any previous history of COVID-19.
On examination, the patient was afebrile. She did not manifest any lymph node enlargement. She had erythematous papules distributed over the face, chest, upper limbs, and thighs and a few non-follicular, pinhead-sized pustules over the forehead [Figure 1]. Her systemic examination did not reveal any abnormality. A Gram stain analysis of a smear prepared from a pustular lesion showed numerous neutrophils and no organisms. Her complete hemogram showed total leukocyte count of 10,800 cells/mm3, neutrophilia, and eosinophilia (neutrophils 78%, lymphocytes 10%, and eosinophils 12%). The absolute eosinophil count was 1296 cells/mm3. Peripheral smear was within normal limits except for neutrophilia and eosinophilia.
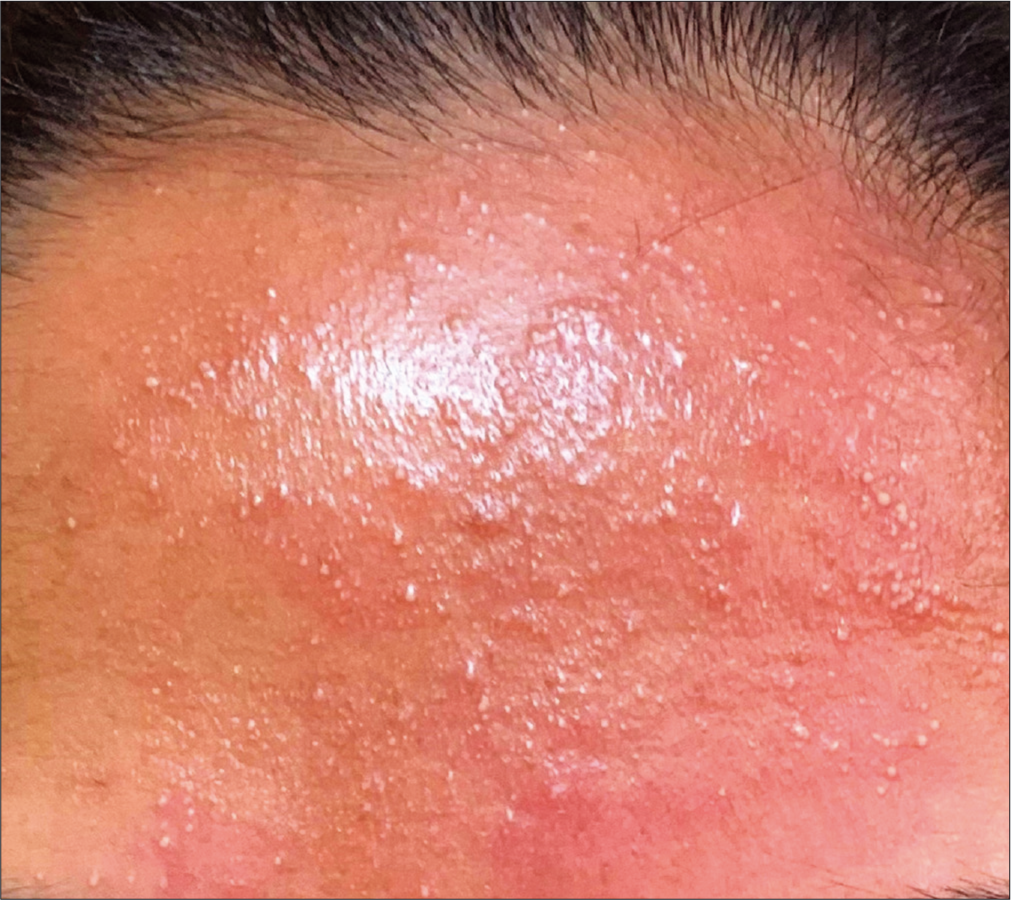
- Pinhead-sized pustules and erythematous papules on the forehead of a patient following Moderna COVID-19 (mRNA-1273) vaccine.
We treated her with cetirizine 10 mg once a day. At the follow-up visit 2 days later, the pustules had increased in number and had extended to the cheeks and upper chest [Figure 2]. She remained afebrile and showed no clinical or laboratory evidence of internal organ involvement. Therefore, the causality assessment was probable on Naranjo adverse drug reaction probability scale [Table 1].[2] We made a final diagnosis of papulopustular rash following the Moderna COVID-19 (mRNA-1273) vaccine.
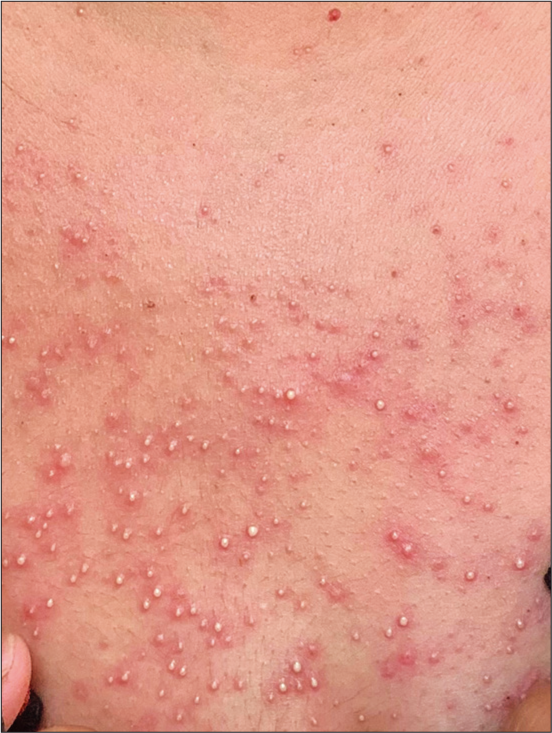
- Pustules on the upper chest of a patient following Moderna COVID-19 (mRNA-1273) vaccine.
| Naranjo adverse drug reaction probability scale | |||||||
|---|---|---|---|---|---|---|---|
| Question | Yes | No | Do not know | Score assigned to the patient who manifested a papulopustular rash followingModerna COVID-19 (mRNA-1273) vaccine | |||
| Are there previous conclusive reports on this reaction | +1 | 0 | 0 | 1 | |||
| Did the adverse event appear after the suspected drug was administered? | +2 | −1 | 0 | 2 | |||
| Did the adverse reaction improve when the drug was discontinued or a specific antagonist was administered? | +1 | 0 | 0 | 1 | |||
| Did the adverse event reappear when the drug was readministered? | +2 | −1 | 0 | 0 | |||
| Are there alternative causes (other than the drug) that could on their own have caused the reaction? | −1 | +2 | 0 | 2 | |||
| Did the reaction reappear when a placebo was given? | −1 | +1 | 0 | 0 | |||
| Was the drug detected in blood (or other fluids) in concentrations known to be toxic? | +1 | 0 | 0 | 0 | |||
| Was the reaction more severe when the dose was increased or less severe when the dose was decreased? | +1 | 0 | 0 | 0 | |||
| Did the patient have a similar reaction to the same or similar drugs in anyprevious exposure? | +1 | 0 | 0 | 0 | |||
| Was the adverse event confirmed by any objective evidence? | +1 | 0 | 0 | 1 | |||
| Total score | 7 | ||||||
9 or more: Definite drug reaction; 5–8: Probable; 1–4: Possible; 0 or less: Doubtful. COVID-19: Coronavirus disease-2019
The patient responded to 5 days of prednisolone (30 mg/day) and cetirizine (10 mg once a day).
DISCUSSION
Strategic Advisory Group of Experts (SAGE) and the World Health Organization had recommended two doses (each of 100 μg/ 0.5 ml) of Moderna COVID-19 (mRNA-1273) vaccine 28–42 days apart in those aged 18 years or more to protect against COVID-19.[3]
One more booster dose was advised for those aged 65 years or more and individuals aged 18–64 years who are at high risk for severe COVID-19 or with frequent institutional or occupational exposure to severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). This third dose was recommended 6 months after the primary vaccination series. On November 19, 2021, the United States of America Food and Drug Administration (US FDA) authorized the use of a single booster dose (after two doses of primary vaccination) for all individuals aged 18 years or more.[3,4]
The vaccine is reported to have an efficacy of 94.1% in protecting against COVID-19 after 14 days of the first dose.[3]
Literature states that a non-serious reaction occurs in about 372 out of every million administered doses of the mRNA vaccines (Moderna and Pfizer-BioNTech vaccines), including injection site pain, fatigue, headache, muscle pain, fever, joint pain, chills, and nausea. The documented adverse events are more frequent after the second dose.[5]
McMahon et al. documented 414 cutaneous reactions after Moderna and Pfizer COVID-19 vaccination, with 83% of them following Moderna.[6] The reaction patterns included delayed large local reactions (most common), local injection site reactions, urticarial eruptions, morbilliform eruptions, pernio/chilblains, cosmetic filler reactions, herpes zoster, flares of herpes simplex, and pityriasis rosea-like reactions.[6] In their series, 21% of patients developed cutaneous reactions following the first dose of vaccine, 63% following the second dose, and 16% had reactions following both doses.[6]
Agaronov et al. reported a 27-year-old Caucasian woman with a history of anaphylaxis following tetracycline who developed acute generalized exanthematous pustulosis (AGEP) within hours of administration of Moderna COVID-19 mRNA vaccine.[7] The rash was accompanied by fever and myalgia.[7] The authors attributed the reaction to vaccine-induced immune activation.
The temporal relation with vaccination, pustular rash, lesions starting on the face (forehead), acute onset and resolution within 15 days, and an elevated neutrophil count (>7000 cells/mm3) favored the possibility of AGEP in our patient.[8] The previous authors had noted a median time interval of 11 days between the onset of drug intake and AGEP when the offender was a non-antibiotic drug.[8] However, our patient did not manifest fever or a flexural predilection for pustular lesions. Moreover, the pustules were limited to a few body sites and those on the chest were larger than the usual tiny, pustules of AGEP.[8]
Based on clinical features, she scored 3 on the AGEP validation score [Table 2]. Histopathology of subcorneal spongiform pustules with or without papillary edema would have earned her a score of 5–6, enough to be classified as probable AGEP [Table 2].[8] With the available workup (a skin biopsy was not performed), we made a diagnosis of vaccine-induced papulopustular rash.
| Feature | Points assigned by the diagnostic score | Points assigned to the patient who manifested a papulopustular rash following Moderna COVID-19 (mRNA-1273) vaccine | |
|---|---|---|---|
| Pustules | Typical +2, compatible with the disease +1, insufficient 0 | 1 | |
| Erythema | Typical +2, compatible with the disease +1, insufficient 0 | 1 | |
| Distribution | Typical +2, compatible with the disease +1, insufficient 0 | 0 | |
| Mucous membrane involvement | Yes - reduce 2 points No 0 |
0 | |
| Acute onset | Yes 0 No - reduce 2 points |
0 | |
| Resolution within 15 days | Yes 0 No - reduce 2 points |
0 | |
| Fever>38°C | Yes +1 No 0 |
0 | |
| Polymorphonuclear leukocytes>7000 cells/mm3 | Yes +1 No 0 |
1 | |
| Histopathology | Other diseases | −10 | Not available |
| Not representative | 0 | ||
| Exocytosis of polymorphonuclear leukocytes | +1 | ||
| Subcorneal or intraepidermal non-spongiform or not otherwise specified pustules with or without papillary edema | +2 | ||
| Spongiform subcorneal and/or intraepidermal pustules with papillary edema | +3 | ||
| Total score of the patient | 3 | ||
Score 1–4: Possible; 5–7: Probable; 8–12: Definite. COVID19: Coronavirus disease2019
Wu and Lin have reported a case of acute localized exanthematous pustulosis (ALEP), a localized variant of AGEP, following the Oxford-AstraZeneca COVID-19 vaccine.[9] This was less likely in our case since the patient had a generalized rash, though the pustules were limited to the face and upper chest.
Although our patient had an elevated absolute eosinophil count, she had no other features (fever/typical rash/internal organ involvement/atypical lymphocytes in peripheral smear) to suggest a diagnosis of drug reaction with eosinophilia and systemic symptoms.[10]
Merrill et al. have observed two men (50 and 80 years, respectively) who developed facial pustular neutrophilic eruption within 24 hours of receiving the first and second doses of the mRNA-1273 SARS-CoV-2 vaccine, respectively.[11] Both patients had facial swelling. Histopathology analysis showed neutrophil infiltration in the interstitium and within the intact follicular epithelium. The lesions responded to topical corticosteroid and topical tacrolimus, respectively. The patient who developed the rash after the first dose of the vaccine did not show any recurrence on receiving the second dose of the same.
Ciccarese et al. reported a 60-year-old woman who developed a papulopustular rosacea-like eruption 4 days after the first dose of Vaxzevria (AstraZeneca) COVID-19 vaccine.[12] The authors also noted a similar eruption 5 days after the second dose of Pfizer-BioNTech COVID-19 vaccine in another 47-year-old woman. Both patients received only sun protection cream and attained a cure in 30 days and 20 days, respectively. The patient who developed the rash after the first dose of the AstraZeneca COVID-19 vaccine did not develop any adverse events following the second dose. The authors compared the rash to the papulopustular eruption associated with epidermal growth factor receptor (EGFR) inhibitors.[13] EGFR inhibitor-induced papulopustular eruption manifests as folliculocentric erythematous papules and pustules that show a preference for seborrheic sites.[13] The pustules in our patient were non-follicular. The typical evolution described for EGFR inhibitor-induced rash (dysesthesia with erythema and edema in the 1st week, an eruption of papulopustular lesions during weeks 2–3, crust formation during weeks 3–4, and persistent erythema, xerosis, and telangiectasia in the affected area lasting 1 month and longer) was lacking in our case. Moreover, unlike the cases reported by Ciccarese et al. who attained a resolution in 20–30 days, our patient showed a rapid resolution of rash.[12]
CONCLUSION
We report this case to improve awareness regarding the varying cutaneous manifestations associated with COVID-19 vaccination.[14,15] Our patient’s course of drug reaction was consistent with the current knowledge of the less severe nature of cutaneous adverse reactions following COVID-19 vaccination.[14]
Declaration of patient consent
Not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Available from: https://www.who.int/news-room/news-updates [Last accessed on 2021 Dec 12]
- A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239-45.
- [CrossRef] [PubMed] [Google Scholar]
- Available from: https://www.who.int/news-room/feature-stories/detail/the-moderna-covid-19-mrna-1273-vaccine-what-you-need-to-know [Last accessed on 2021 Dec 12]
- Available from: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-expands-eligibility-covid-19-vaccine-boosters [Last accessed on 2021 Dec 16]
- Available from: https://www.nature.com/articles/d41586-021-00290-x [Last accessed on 2021 Dec 18]
- Cutaneous reactions reported after moderna and Pfizer COVID-19 vaccination: A registry-based study of 414 cases. J Am Acad Dermatol. 2021;85:46-55.
- [CrossRef] [PubMed] [Google Scholar]
- Acute generalized exanthematous pustulosis induced by moderna COVID-19 messenger RNA vaccine. JAAD Case Rep. 2021;16:96-7.
- [CrossRef] [PubMed] [Google Scholar]
- Acute generalized exanthematous pustulosis (AGEP)-a clinical reaction pattern. J Cutan Pathol. 2001;28:113-9.
- [CrossRef] [PubMed] [Google Scholar]
- Oxfor-AstraZeneca COVID-19 vaccine-induced acute localized exanthematous pustulosis. J Dermatol. 2021;48:e562-3.
- [CrossRef] [Google Scholar]
- Reply to: 'Using a diagnostic score when reporting the long-term sequelae of the drug reaction with eosinophilia and systemic symptoms. J Am Acad Dermatol. 2013;69:1060-2.
- [CrossRef] [PubMed] [Google Scholar]
- Association of facial pustular neutrophilic eruption with messenger RNA-1273 SARS-CoV-2 vaccine. JAMA Dermatol. 2021;157:1128-30.
- [CrossRef] [PubMed] [Google Scholar]
- Two cases of papulo-pustular rosacea-like eruptions following COVID-19 vaccinations. JEADV. 2021;35:e868-70.
- [CrossRef] [Google Scholar]
- Cutaneous adverse reactions specific to epidermal growth factor receptor inhibitors. J Med Life. 2015;8:57-61.
- [Google Scholar]
- COVID-19 vaccines and the skin. The landscape of cutaneous vaccine reactions worldwide. Dermatol Clin. 2021;39:653-73.
- [CrossRef] [PubMed] [Google Scholar]
- Herpes zoster following ChAdOx1 nCoV-19 corona virus vaccine (recombinant): A case report. J Skin Sex Transm Dis 2021:1-3.
- [CrossRef] [Google Scholar]






