Translate this page into:
Rituximab-induced vasculitis: A rare occurrence
*Corresponding author: Anusha P, Department of Dermatology, Venereology and Leprosy, Government Medical College, Kottayam, Kerala, India. anushasivanunni@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Anusha P, Santhosh AR, Trikkovil AM, Jayalakshmi S, Celine MI, Vineetha M, et al. Rituximab-induced vasculitis: A rare occurrence. J Skin Sex Transm Dis 2024:6:47-9. doi: 10.25259/JSSTD_72_2023
Abstract
Rituximab is a chimeric murine/human monoclonal antibody that targets the CD20 antigen expressed on B lymphocytes. It is Food and Drug Administration-approved for use in pemphigus vulgaris (PV). Usual adverse effects are mild, and vasculitis has been reported very rarely. There are only three biopsy-proven case reports of rituximab-induced vasculitis in medical published literature and none in pemphigus. Here, we report a case of rituximab-induced vasculitis in a 46-year-old female with PV.
Keywords
Rituximab
Vasculitis
Pemphigus Vulgaris
INTRODUCTION
Rituximab is a chimeric murine/human monoclonal antibody that targets the CD20 antigen expressed on B lymphocytes.[1] The drug was originally developed for the treatment of B-cell neoplasms, but now it is also Food and Drug Administration-approved for use in autoimmune diseases such as rheumatoid arthritis (RA), granulomatosis with polyangiitis (GPA), microscopic polyangitis (MPA), and pemphigus vulgaris (PV).[2] Following administration of rituximab a variety of adverse events can occur, but are usually mild and infusion related.[1] However, rarely serum sickness, Steven-Johnson syndrome (SJS), proinflammatory syndrome mimicking acute RA, vasculitis, and transaminitis are also reported.[1,3] We report a case of biopsy-proven leukocytoclastic vasculitis following rituximab administration in a 46-year-old female with PV.
CASE REPORT
A 46-year-old female patient with PV and on systemic steroids and mycophenolate mofetil was started on rituximab infusion due to the development of metabolic side effects of steroids. The patient was stable during and immediately post-infusion. However, within 24 h of rituximab administration, she developed multiple, discrete, and confluent palpable and non-palpable purpura over the upper and lower limbs and the anterior abdomen and chest [Figures 1a-b]. There were no systemic symptoms. General and systemic examination was within normal limits. Investigations showed hemoglobin – 13 g/dL, leukocyte count of 9400 cells/mm3 (neutrophil 81%, lymphocyte 15%, mixed 3%), platelet count – 1.75 lakh/mm3, erythrocyte sedimentation rate – 66 mm/1st h, C-reactive protein – 4.5 mg/dL, prothrombin time – 12.6, international normalized ratio – 0.94, and activated partial thromboplastin time – 26.4. Urine routine examination was normal. Hepatitis B surface antigen and anti-hepatitis C virus antibodies were negative. Punch biopsy from the most recent purpuric lesion over the left leg showed fibrinoid necrosis and neutrophilic infiltration in dermal capillaries, which were consistent with neutrophilic small vessel vasculitis [Figures 1c-d]. Due to the absence of infection or other vasculitis triggers and the temporal correlation with rituximab infusion, the patient received a diagnosis of rituximab-induced vasculitis and discontinued further rituximab therapy. Lesions were completely resolved within a week without any sequelae.
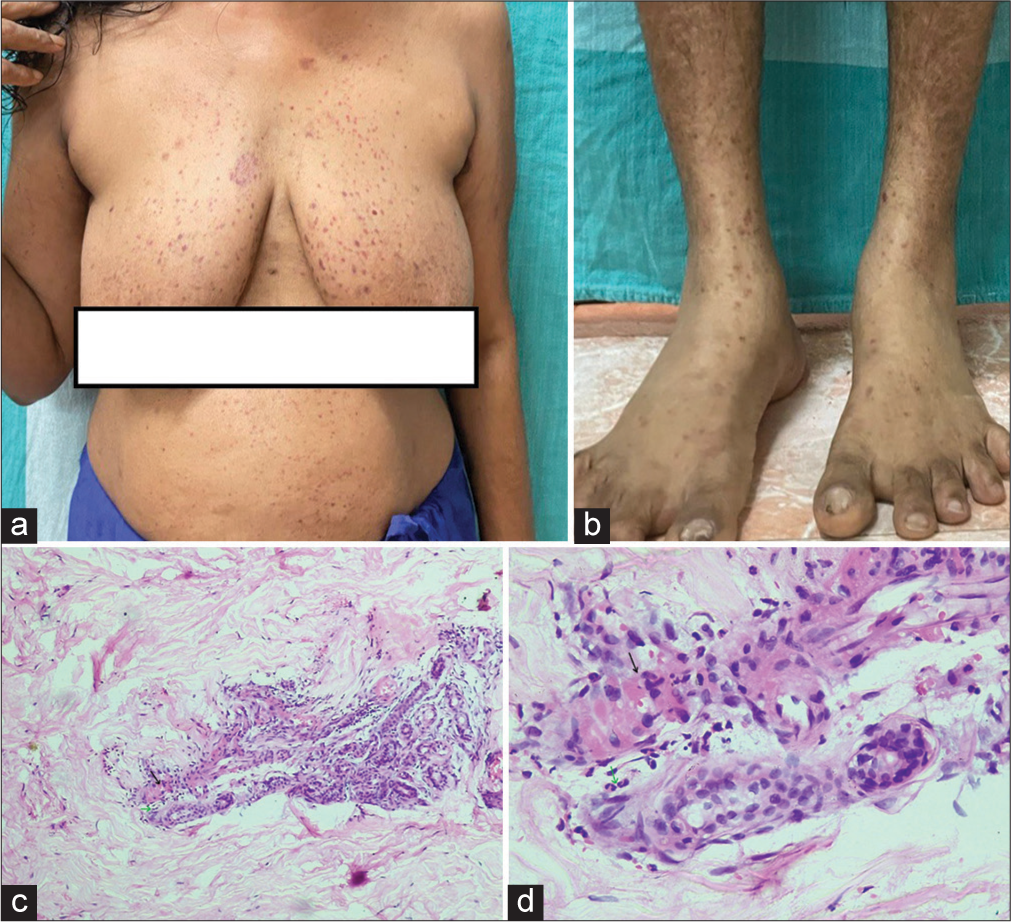
- (a): Multiple palpable and non-palpable purpuric lesions over chest and abdomen; (b): Multiple purpuric lesions over the leg and foot; (c): Haematoxylin and Eosin (H&E) × (10) showing inflammatory infiltrates (green arrow) and fibrinoid necrosis (black arrow) in and around the vessel wall; (d): H&E × (40) showing polymorphonuclear leukocytic inflammatory infiltrates (green arrow) and fibrinoid necrosis (black arrow) in and around the vessel wall.
DISCUSSION
Vasculitis is a rarely reported adverse reaction of rituximab though rituximab is approved for use in systemic vasculitis. Common drugs inducing vasculitis include antibiotics, antithyroid drugs such as methimazole, carbimazole, propyl thiouracil, and anti-tumor necrosis factor-alpha (TNF-α) agents. Propyl thiouracil can induce anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis.[4]
O’Dereure et al. described a case of generalized cutaneous vasculitis in a patient with chronic lymphocytic leukemia, which occurred two days after receiving a 700 mg dose of rituximab with a similar presentation to our case. The patient was managed symptomatically.[5] Tezer et al. reported a similar adverse reaction along with arthritis in a patient treated for myasthenia gravis ten days after a 1 g infusion of rituximab, and he was treated with systemic steroids.[6] Kandula and Kouides report another patient with non-Hodgkin’s lymphoma who developed vasculitis after the third dose of rituximab injection and was symptomatically managed.[7] There are no reports of similar occurrences in patients with pemphigus. Our patient developed symptoms within 24 hours of the first infusion without systemic symptoms, and lesions resolved in a week. Severe cutaneous adverse reactions are reported in 2% of patients who are treated with rituximab, which includes SJS, toxic epidermal necrolysis, and systemic inflammatory response syndrome. Other side effects include pruritus, urticaria, serum sickness, vesiculobullous lesions, and lichenoid eruption. The onset of lesions varied from 1 to 13 weeks after exposure.[8] The exact pathophysiological mechanism behind rituximab-induced vasculitis is still unknown. The suggested explanation includes rituximab– anti-rituximab antibody complex deposits and the cytokine release syndrome induced by rituximab, which involves mainly TNFα and interleukin-6.[1] However, the rare occurrence and the variable incubation period between rituximab administration and the appearance of vasculitis in the reported cases make it difficult to establish the pathophysiology. Skin biopsy is diagnostic, and there are no pathological or laboratory markers for differentiating between drug-induced vasculitis from other causes. Drug-induced vasculitis can present with clinical features similar to those of cutaneous small vessel vasculitis. Early recognition and discontinuation of the offending drug are essential to prevent further damage to vital organs. Diagnosis of drug-induced vasculitis can be considered if there is a temporal association between the signs and symptoms of an offending drug and regression of lesions on discontinuing the drug. Other potential etiologies of vasculitis should be ruled out, and any signs of systemic involvement should be thoroughly evaluated.[3] Treatment is by withdrawing and avoiding rechallenge of the offending drug and the same class of drugs. In mild cases, withdrawal of the offending drug will improve symptoms, and a short course of systemic steroids is indicated if there is systemic involvement.[3]
CONCLUSION
Rituximab-induced vasculitis is rare. In any patient who develops vasculitis following rituximab administration, this adverse reaction should be considered, and further administration of this drug should be avoided if it is suspected as the cause of vasculitis.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
Dr Mary Vineetha is on the editorial board of the Journal.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Rituximab-induced vasculitis: A case report and review of the medical published work. J Dermatol. 2009;36:284-7.
- [CrossRef] [PubMed] [Google Scholar]
- Rituximab: A review in pemphigus vulgaris. Am J Clin Dermatol. 2020;21:149-56.
- [CrossRef] [PubMed] [Google Scholar]
- Rituximab induced transaminitis in pemphigus foliaceus. J Skin Sex Transm Dis. 2019;1:45-7.
- [CrossRef] [Google Scholar]
- Drug-induced vasculitis: A clinical and pathological review. Neth J Med. 2012;70:12-7.
- [Google Scholar]
- Rituximab-induced leukocytoclastic vasculitis. Neurol Sci Neurophysiol. 2022;39:161-3.
- [CrossRef] [Google Scholar]
- Rituximab-induced leukocytoclastic vasculitis: A case report. Arch Dermatol. 2006;142:246-7.
- [CrossRef] [PubMed] [Google Scholar]
- A review of rituximab in cutaneous medicine. Dermatol Online J. 2006;12:3.
- [CrossRef] [PubMed] [Google Scholar]






