Translate this page into:
The clinical, dermoscopic, and histopathologic differentiation of cutaneous leishmaniasis from cutaneous sarcoidosis and tuberculosis: A review article
*Corresponding author: Jacob Al-Dabbagh, Cancer Research Center, Tishreen University, Latakia, Syrian Arab Republic. jacobaldabbagh0@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Al-Dabbagh J, Ismail N. The clinical, dermoscopic, and histopathologic differentiation of cutaneous leishmaniasis from cutaneous sarcoidosis and tuberculosis: A review article. J Skin Sex Transm Dis 2024:6:13-21. doi: 10.25259/JSSTD_55_2023
Abstract
Leishmaniasis is a neglected tropical disease caused by infected female sandflies (Phlebotomus and Lutzomyia), which are vector-borne protozoan parasites belonging to the genus Leishmania. The diagnosis of cutaneous leishmaniasis (CL) can be challenging and delayed, particularly in areas where leishmaniasis is not endemic. CL is known as “the great imitator” because it can mimic many skin disorders due to its various clinical manifestations. Cutaneous sarcoidosis (CS) and cutaneous tuberculosis (CTB), which are also known as “the great imitators,” should be differentiated from CL due to the common clinical, dermoscopic, and histopathologic features. In this article, we aim to help clinicians differentiate CL by listing its clinical manifestations those are similar to CS and CTB and highlighting common and uncommon dermoscopic and histopathologic findings. We have also created a brief approach to diagnose CL, CS, and CTB, which is presented as a diagram. A search was performed on PubMed and Google Scholar using the keywords CL, CTB, CS, and granulomatous disease for all articles, with no restrictions. Updated articles on leishmaniasis, tuberculosis, and sarcoidosis, including some new concepts in clinical presentations, dermoscopy, and histopathology, were reviewed.
Keywords
Leishmaniasis
Tuberculosis
Sarcoidosis
Dermoscopy
Histopathology
INTRODUCTION
Leishmaniasis is a vector-borne disease that manifests as a cutaneous, mucosal and visceral disease.[1] It is caused by Leishmania parasites and transmitted through the bite of sandflies belonging to Phlebotomus spp. and Lutzomyia spp.[2]
Leishmania are classified into two types, according to the Eurocentric worldview, which is Old World species (Leishmania major, Leishmania tropica, Leishmania donovani, Leishmania infantum, and Leishmania aethiopica) and New World species (Leishmania amazonesis, Leishmania mexicana, Leishmania venezuelensis, Leishmania chagasi, Leishmania naiffi, and Leishmania viannia subgenus including Leishmania braziliensis, Leishmania panamensis, and Leishmania guyanesis).[1,3]
The majority of countries in the Americas, the Middle East, the Mediterranean basin, and Central Asia are affected by leishmaniasis (approximately 95% of cases).[1,4] The Old World leishmaniasis occurs in the Eastern Hemisphere, including Asia, the Middle East, Africa, and Southern Europe.[1] On the other hand, New World leishmaniasis occurs in the Western Hemisphere, especially in Mexico, Central America, South America, and the United States.[1] Poor hygiene, poverty, migration, malnutrition, poor housing conditions, and immunocompromised status are risk factors for leishmaniasis.[1,5]
Cutaneous leishmaniasis (CL) can mimic many skin disorders, such as cutaneous sarcoidosis (CS) and cutaneous tuberculosis (CTB), which are considered “great imitators” due to the variety of their clinical presentations.[5-7] These “great imitators” belong to cutaneous granulomatosis and have common clinical, dermoscopic, and histopathologic features, which make it difficult for physicians to differentiate these diseases from each other and can lead to inappropriate treatment and associated morbidities.[5,6,8,9]
CLINICAL PRESENTATION
CL, mucosal leishmaniasis (ML), and visceral leishmaniasis (VL) are the three main phenotypic classifications of the disease. Leishmaniasis can present with an array of different clinical manifestations. The spectrum of clinical diseases can be classified to include CL of the Old World, CL of the New World, diffuse CL, disseminated CL, post-kala-azar CL, ML, VL, and leishmaniasis recidivans (LR).[1]
It is usually typical to diagnose CL in endemic regions by specific clinical features.[10] Depending on the type and stage of CL, papules, nodules, plaques, or ulcers can be the presenting manifestations.[10] Acute, subacute, and chronic CL may all be present in some individuals, and it can also be asymptomatic or subclinical.[1]
CS also has various manifestations ranging from more distinctive lesions (including papules, nodules, plaques, lupus pernio [LP], and infiltrative scars) to less distinctive lesions (including Darier-Roussy disease, scarring and nonscarring alopecia, erythroderma, annular, hypopigmented, angiolupoid, atrophic, ulcerative, psoriasiform, and ichthyosiform lesions).[6] In addition, the clinical manifestations of CTB are varied and include papules, plaques, macules, patches, nodules, abscesses, erosions, and ulcers that mimic diverse skin disorders.[7]
THE CLINICAL MANIFESTATIONS AND DIFFERENTIAL DIAGNOSIS OF CL
Typically, CL lesions are classified into two categories, Old World and New World, according to the geographic area.[1,5] The lesions usually begin at the location of the sandfly bite as a small, single, and non-suppurative papule (typically on well-exposed locations of the face and limbs).[1,5] However, numerous lesions may also occur.[1] The papule slowly evolves over weeks or months into a nodule that can develop into a painless ulcer with a piled-up border.[1,5] Depending on the respective species, these ulcers may spontaneously heal over months or years or can cause disfigurement and scars.[1,5] It is estimated that progression, chronicity, and more severe clinical presentations occur in up to 10% of CL cases.[5]
CL and CS have common clinical presentations.[5] In CS, papules are the most common lesions, which are discrete and typically located on the face, eyelids, and nasolabial folds.[6] These papular lesions are more frequent in acute sarcoidosis, and they heal without causing scars.[6] The sarcoid lesions can be red, brownish-red, brown, or violet.[6] Ulcerations can occur independently of other CS lesions or as a result of the worsening of other forms, such as papulonodular or atrophic lesions.[6]
Tuberculosis can also resemble CL due to its varied manifestations, including scrofuloderma, tuberculosis verrucosa cutis (TVC), erythema induratum of Bazin, and tuberculosis chancre.[7,9] Erythema induratum of Bazin manifests as erythematous-purple subcutaneous nodules, typically on the thighs and legs.[9] The nodules progress a few centimeters, resulting in deep ulcers with caseous discharges and a pigmented scar, whether treatment is successful or not.[9]
TVC lesions are presented as single and painless lesions that can range in appearance from erythematous papules to verrucous plaques that extend to the peripheral areas. Tuberculous chancre, an exogenous form of tuberculosis, results from direct skin infections caused by mycobacterium tuberculosis, which can occur from tattoos, body piercings, traumatic injuries, and surgical procedures performed with non-sterile materials. The tuberculous chancre present as firm, painless, slow-growing papules or nodules that are reddish-brown. The lesions develop into friable ulcers within 2 to 4 weeks, with a tendency to bleed.[9] Scrofuloderma is a common form of CTB; caused by direct expansion from an underlying tuberculous focus in lymph nodes, bones, joints, or testicles. The common locations include neck, axillae and groin, with the cervical lymph nodes as a frequent infection source. Initial lesions present as red-brown, firm, painless, and subcutaneous nodules that develop into ulcers. There is a possibility of spontaneous healing, resulting in retractions, keloid scars, and the atrophic sequel.[9]
Chronic lupoid leishmaniasis
CL is considered chronic if it persists for more than two years.[5] Chronic CL lesions may not ulcerate and can be treatment resistant.[5] Chronic lupoid leishmaniasis is another rare manifestation of Old World CL that has clinical and histopathologic features similar to lupus vulgaris (LV), the most common form of CTB, which poses a diagnostic challenge.[5,11]
The common clinical characteristic of LV is a flat and red-brown papulotubercular lesion, frequently located on the legs and buttocks, which ultimately combine to form a plaque.[9,11]
The plaque expands gradually peripherally, exhibiting atrophy and discoloration in its center with a verrucous or serpiginous border.[9,11] The typical appearance of LV is referred to as “apple jelly nodules” by diascopy.[9]
LV occurs most frequently on the face and has the highest potential for disfigurement among the other forms of CTB.[7,11]
LP should also be taken into account as a differential diagnosis of LR and chronic lupoid leishmaniasis.[5] LP is characterized by papulonodules and plaques that mainly affect cold-affected areas such as the nose, ears, and cheeks.[6] Without treatment, the lesions persistently infiltrate and indurate, eventually eroding the underlying bone and cartilage and causing extensive damage and disfigurement.[6] Women and African Americans are more frequently affected by LP.[6]
LR
LR is a rare manifestation of Old World CL, which is associated most frequently with L. tropica and describes the formation of new papular lesions either during or following the healing of the acute lesions.[1,5] The most common clinical manifestations are scaly and erythematous papules surrounding old scars or healed lesions. They can persist isolated or in clusters and enlarge over many years.[1,5] LR, LP, and LV can be difficult to differentiate from each other.[5] On diascopy, the peripheral papules in LR may show apple-jelly color.[5] However, LR is not destructive.[5]
Diffuse CL
Diffuse CL presents clinically as diffuse skin lesions with non-ulcerative plaques and nodules located on the face and limbs.[1,5] The differential diagnosis of diffuse CL includes LV and post-kala-azar dermal leishmaniasis.[10]
Disseminated leishmaniasis
Immunosuppression is often associated with disseminated leishmaniasis, which is typically linked to ulceration or mucosal involvement, and characterized by numerous noncontiguous pleomorphic lesions that are resistant to treatment and imitate classic CL lesions.[1,5]
Other atypical forms of leishmaniasis
There are various atypical cutaneous manifestations of leishmaniasis, including erysipeloid, psoriasiform, eczematous, verrucous, discoid lupus erythematosus-like, annular, acneiform, paronychial, fissure leishmaniasis, zosteriform leishmaniasis, chancriform, panniculitic, sporotrichoid, and palmoplantar.[1,5]
Erysipeloid leishmaniasis
The erysipeloid form of CL is characterized by chronic, painless, diffuse, erythematous, asymmetric, and infiltrated plaques located on the face (cheeks and nose). These lesions are typically not ulcerated and vary in degrees of scaling. The erysipeloid CL mainly affects middle-aged or elderly women without the involvement of lymphadenopathy or mucous membranes. The occurrence of this form may be caused by prolonged sun exposure or post-traumatic cutaneous lesions.[5]
Psoriasiform leishmaniasis
Similar to psoriasis, CL can manifest as erythematous, scaly lesions and hyperkeratotic plaques. Psoriasiform CL lesions presenting as infiltrated plaques covered by scales and crusts usually form at a single location and extend peripherally. HIV patients tend to develop more psoriasiform CL lesions.[5]
Psoriasiform leishmaniasis should be differentiated from psoriasiform CS and psoriasiform CTB.[6,7] Psoriasiform sarcoidosis lesions present as plaques that mimic psoriasis, but they resolve with hypopigmentation, scarring, or atrophy.[6] Psoriasiform CS is mainly reported in dark-skinned people.[6]
Eczematous leishmaniasis
Eczematous leishmaniasis can present as eczema-like lesions on the dorsum of hands and feet or nummular eczema-like lesions on the limbs with a possible occurrence of pruritus.[5] HIV patients are more likely to develop eczematoid CL lesions.[5] Eczematous CTB should be considered as a differential diagnosis of eczematous leishmaniasis.[7] Eczematous CTB, usually paucibacillary, may also mimic lichen simplex chronicus with severe and chronic pruritus.[7]
Verruciform leishmaniasis
Verruciform CL is an uncommon clinical form of CL.[12] It should be differentiated from verrucous sarcoidosis, which usually occurs in patients who have a long-standing systemic disease, and TVC.[6,11] TVC, the typical exogenous form of tuberculosis, begins as a small and painful papule surrounded by a purple and inflammatory corona that develops into an asymptomatic warty lesion.[9,11] Moreover, psoriasiform CTB has also been noted in TVC.[7] TVC is more prevalent in physicians, anatomists, limbs prone to traumatic injuries, and barefoot children living in tropical regions because the infection spreads through an injured dermal layer.[9] In addition, individuals who were previously infected with tuberculosis and have moderate to high immunity may develop TVC.[12]
Discoid lupus erythematosus-like CL
CL may appear with a butterfly distribution on the face and mimics discoid lupus erythematosus lesions. The appearance of atrophic plaques, peripheral papules, and central scale in discoid lupus erythematosus-like CL can be misdiagnosed as LR.[5]
Plaque sarcoidosis, which manifests as indurated and discrete plaques, can mimic discoid lupus if it is located on the face with central scaling and atrophy.[6] LV should be differentiated from discoid lupus erythematosus-like CL.[7] Both of these diseases are most frequently seen on the face and have prolonged courses.[7]
DERMOSCOPY
Granulomatous disorders may have common dermoscopic features, but a detailed and accurate evaluation of each feature and its pattern may be helpful in distinguishing between them. The presence of structureless orange or orange-yellowish (focal or diffuse) areas and vessels, which can be linear or branching, represent the dermoscopic findings of granulomatous disorders. However, neither the presence nor the absence of orange to orangish-yellow structureless areas is adequate to confirm the diagnosis. Other dermoscopic findings of granulomatous disorders can be observed, including scaling, erythema, milia-like cysts, whitish areas, follicular plugs, and pigmentation structures.[13]
The dermoscopy of CL
Dermoscopic evaluation of CL revealed generalized erythema, vascular structures, and yellow tear-like structures.[13,14]
The lateral compression of the follicular ostium, which is caused by tumoral growth, results in follicular keratin plugging, which corresponds to yellow tear-like structures on dermoscopy.[13] The vascular structures can manifest with combinations of dotted, comma-shaped, tree-like, hairpin, glomerular, irregular linear, atypical, strawberry pattern, and polymorphous vessels.[13-15]
More progressed lesions show a white starburst-like pattern, central erosion, and peripheral vascular pattern.[14] Other remarkable features include scaling, pustules, salmon-colored ovoid structures, white scar-like areas, milia-like cysts, crust, follicular plugs, and perilesional hypopigmented halos.[13,15-17]
The dermoscopy of CS
The dermoscopic findings of CS include translucent yellow to orange globules and/or structureless areas, which may be focal or diffuse, with various morphologies of vessels.[6,14] Other findings, such as white scar-like areas, pigmentation structures, milia-like cysts, follicular plugs, and scales, can also observed.[13,14]
The various morphologies of vessels can be exhibited as linear, dotted, branching, and glomerular vascular patterns.[13] The vessels appear sharper and well-focused as a result of the granulomas pushing them towards the surface.[13]
The dermoscopy of CTB
The dermoscopic presentation of CTB, except for LV, is not comprehensively described in English literature.[18] The presence of yellowish-orange structureless (focal or diffuse) areas and focused linear or branching vessels are noticed on the dermoscopic examination of LV.[13,14] In addition, scales, pigmentation structures, follicular plugs, whitish reticular streaks, and milia-like cysts can be seen in LV.[13]
We summarized the above-mentioned dermoscopic findings of CL, CS, and CTB by comparing them in Table 1.[4,13-17,19]
| Dermoscopic findings | Cutaneous leishmaniasis | Cutaneous sarcoidosis | Cutaneous tuberculosis |
|---|---|---|---|
| Generalized erythema | ✓ | X | X |
| Vascular structures | ✓ | ✓ | ✓ |
| Orange-yellowish structureless areas | ✓ | ✓ | ✓ |
| yellow to orange globules areas | X | ✓ | X |
| yellow tear-like structures | ✓ | X | X |
| White scar-like areas | ✓ | ✓ | ✓ |
| White starburst-like | ✓ | X | X |
| Whitish reticular streaks | X | X | ✓ |
| Salmon-colored ovoid structures | ✓ | X | X |
| Perilesional hypopigmented halos | ✓ | X | X |
| Follicular plugs | ✓ | ✓ | ✓ |
| Ulcer crust | ✓ | X | X |
| Pigmentation structures | X | ✓ | ✓ |
| Milia-like cysts | ✓ | ✓ | ✓ |
| Scales | ✓ | ✓ | ✓ |
| Pustules | ✓ | X | X |
HISTOPATHOLOGY
Histopathologic examination is an essential diagnostic tool that can assist in distinguishing between diseases that are mimickers of CL.[5] The histopathology of CL shows granulomatous findings similar to those of cutaneous disorders with different etiologies, such as CTB and CS.[9,20]
The histopathology of CL
The histopathologic features of leishmaniasis differ from well-defined/ill-defined granulomas to inflammatory infiltration with necrosis and non-necrosis.[1,21] Early stages of CL are identified by a diffuse and dense dermal infiltrate of parasitized histiocytes, lymphocytes, plasma cells, and varying numbers of neutrophils.[5] In early lesions of Old World CL, amastigotes can be identified in 50–70% of skin biopsies.[5] Amastigotes decrease as ulcers become more chronic.[5] In addition, an uninvolved papillary dermis (Grenz zone) may exist.[5] In the upper dermis, epithelioid cell granulomas with giant cells develop as the lesions progress.[5] Small tuberculoid granulomas begin to replace the declining number of parasitized histiocytes as chronicity develops.[5]
It becomes more challenging in the latter stages of CL to confirm the diagnosis, when granulomas dominate and parasite-filled histiocytes gradually disappear.[21] In chronic relapsing CL, which is also known as LR, infections appear within a scar left by a prior primary acute cutaneous leishmanial infection.[10]
If Grenz zone is absent, the histopathologic examination can reveal pseudoepitheliomatous hyperplasia and variable epidermal changes.[10] The epidermis may likewise have hydropic degeneration of the basal cell layer and a deficiency of pigment with a broad lymphocytic superficial and deep dermal infiltration.[10] In chronic relapsing CL, only a few amastigotes are present, making the histologic diagnosis difficult.[10] The histopathologic examination of diffuse CL has distinct features.[10] The dermis exhibits a diffuse infiltrate of macrophages containing amastigotes.[10] A large vacuole in the cytoplasm of heavily parasitized macrophages is surrounded by few lymphocytes, normally seen in people who have an early immune response or anergic diffuse CL.[10] In addition, there is a mixed inflammatory infiltrate without necrosis.[10] The histological spectrum of CL can be classified based on the modified Ridley’s pattern, as shown in Table 2.[22]
| Group | Histopathologic response |
|---|---|
| I | A skin biopsy appears normal, with patches of collagen degeneration |
| II | Dominant severe necrotizing process in the dermis |
| III | A diffuse and severe inflammatory infiltrate dominates the dermis |
| IV | Scattered Langhans giant cells and primitive epithelioid histiocytes |
| V | Well-formed granulomas and well-developed epithelioid histiocytes |
The histopathology of CS
The histopathologic features of CS include non-caseating granulomas, also known as “naked granulomas,” which are compact clusters of epithelioid macrophages and multinucleated giant cells, with minimum to no central necrosis surrounded by a scattered infiltrate of lymphocytes and occasionally plasma cells.[23,24]
The atypical histopathologic findings of CS include the existence of foreign material, necrosis, interstitial and peri adnexal distribution of granulomas, the concurrence of granulomatous and lichenoid infiltrate, granulomatous vasculitis, and epidermal changes.[6]
Non-caseating granulomas are not specified for CS and are noticed in other disorders, such as leishmaniasis.[6]
The histopathology of CTB
The histopathology of CTB is characterized by caseating ill-formed granulomas with intense inflammatory reaction and may be positive for acid fast-bacilli.[25]
The clinical variants of CTB have a similar histological basis, which includes lymphocytes, epithelioid histiocytes, and giant cells.[26] The host’s varied ability to organize the granulomatous process is the cause of the histological variations seen in each clinical variant.[26] The histopathology of CTB can be divided into three categories to affirm the idea that the intensity of the host’s immune response is the cause of the disease’s clinical and pathological presentation.[26] These categories are demonstrated in Table 3.[26]
| The histopathologic categories | The histopathologic findings |
|---|---|
| I- Well-formed granulomas with an absence of caseous necrosis | |
| A-Lupus vulgaris | Acanthosis, papillomatosis, and pseudoepitheliomatous hyperplasia can be seen in either an atrophic or hypertrophic epidermis. Well-formed tuberculous granulomas in the reticular dermis are frequently associated with Langhans giant cells or foreign body-like granulomas. The lymphocytic infiltrate is dense, and caseous necrosis is rare, but it can present in small foci central to the granuloma. Sarcoidosis-like granulomas may be observed. Acid-fast bacilli are present infrequently. |
| B-Lichen scrofulosorum | Presence of epithelioid granulomas in the dermis, which are located more superficially adjoining to the adnexa and surrounded by lymphocytes. Giant cells are absent in general. Acid-fast bacilli and caseous necrosis are absent. |
| II-Intermediate forms: Granulomas with caseous necrosis | |
| A-Tuberculosis verrucosa cutis | Hyperkeratosis, acanthosis, and papillomatosis are prominent as epidermal changes. In the dermis, caseous necrosis of moderate intensity and tuberculous granulomas are present simultaneously. Also, bacilli may be present. |
| B-Primary cutaneous tuberculosis | It differs depending on the time of inoculation. Necrotizing neutrophil infiltrate containing numerous acid-fast bacilli are present in recent lesions. Granulomas become more organized, and the number of bacilli decreases at a later stage. |
| C-Acute miliary tuberculosis | Nonspecific inflammatory infiltration with rich plasma cells and lymphocytes exists. If caseous necrosis is present, it will be focal, and microabscesses may occasionally be noticed. The severity of the condition directly varies with the presence of bacilli. |
| D-Tuberculosis orificialis | There are tuberculoid granulomas, and superficial ulcers associated with caseous necrosis in the deep dermis. |
| E-Papulonecrotic tuberculid | Presence of dermal necrosis with granulomatous infiltrate, leukocytoclastic vasculitis, perivascular edema, or suppurative follicular necrosis. |
| III-Poorly formed granulomas with intense caseous necrosis | |
| A-Scrofuloderma | Massive central necrosis is accompanied by suppuration, forming an abscess in many cases. Granulomas are visible as traces at the periphery of the lesion. Mycobacterium tuberculosis can also be found in this location. |
| B-Metastatic abscesses and gumma | Giant cells and macrophages surround abundant caseous necrosis with central ulceration. Acid-fast bacilli are commonly observed. |
We summarized the above-mentioned histopathological findings of CL, CS, and CTB by comparing them in Table 4.[1,5,6,9,10,21,23-26]
| Histopathological findings | Cutaneous leishmaniasis | Cutaneous sarcoidosis | Cutaneous tuberculosis |
|---|---|---|---|
| Epidermis | |||
| Epidermal changes | + | +1/− | +/− |
| Parasites | +2/− | - | - |
| Dermis | |||
| Parasites | +2/− | - | - |
| Inflammatory infiltration | +/− | +/− | +/− |
| Necrosis | +/− | +/− | +/− |
| Caseous | - | - | +/− |
| Granulomas | |||
| Ill-formed | +/− | +/− | +/− |
| Well-formed | +/− | +/− | +/− |
| Sarcoidal3 | +4/− | + | +5/− |
THE DIAGNOSTIC APPROACH
Although the diagnosis of CL can be made clinically with direct (microscopy, histopathology, and parasite culture) and/or indirect (serology, molecular, and immunologic tests) confirmations, the diagnosis of leishmaniasis is considered challenging.[1,5] CL should be suspected based on the patient’s history, epidemiology, clinical symptoms, and physical examination findings.[1] However, clinical diagnosis is not considered adequate and should always be supported with laboratory testing.[27]
CS may have diverse presentations and can affect multiple systems.[23] The diagnosis of CS is typically based on the clinical presentation and histopathologic confirmation of non-caseating granulomas with the support from history, physical examination, laboratory investigations, chest radiography, pulmonary function testing, fundoscopic and ophthalmologic evaluation, and electrocardiography to exclude the systemic involvement.[6,23] The diagnosis of CS is confirmed only after excluding other suspected diseases.[23]
It is difficult to distinguish sarcoidosis from tuberculosis, particularly in countries with a high tuberculosis prevalence.[25,28] Systemic symptoms such as fever, weight loss, malaise, and fatigue can present in both sarcoidosis and tuberculosis.[28] Therefore, the accurate diagnosis of tuberculosis requires a detailed evaluation of clinical, radiologic, and laboratory findings.[25,28]
We created a schematic diagram for a stepwise approach for the diagnose CL [Figure 1].[1,5,8,20,23,25,26,28]
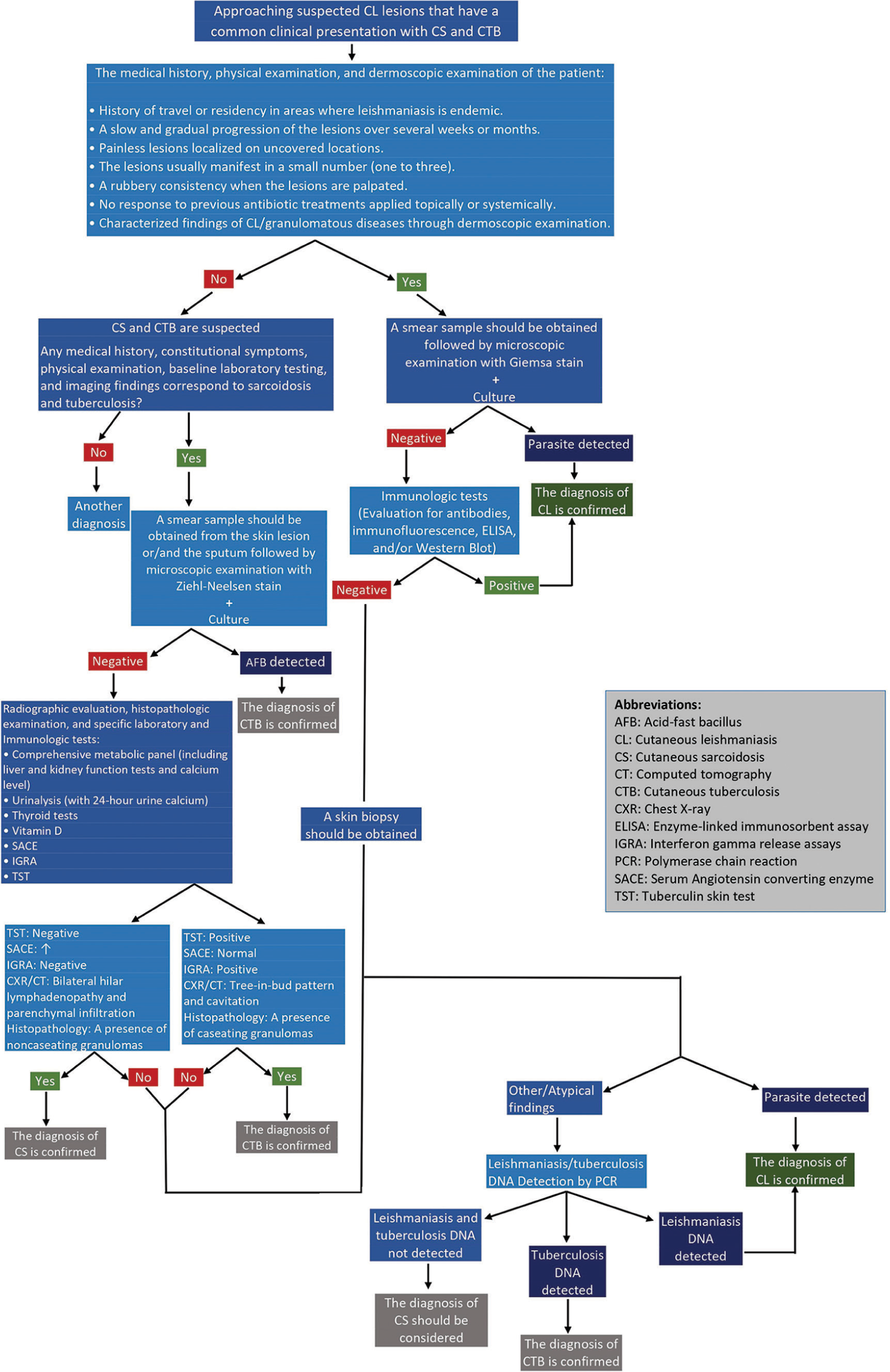
- A schematic diagram in approaching the diagnosis of cutaneous leishmaniasis by excluding cutaneous sarcoidosis and tuberculosis if they are considered differential diagnoses.
CONCLUSION
CL may be difficult to diagnose because it imitates infectious, malignant, and granulomatous diseases. Among granulomatous diseases, CS and CTB should be differentiated from CL as they have common clinical, histopathologic, and dermoscopic characteristics.
In case the medical history of the patient suggests the diagnosis of CL but the performed diagnostic methods did not detect the parasite and the physical examination is similar to CS and CTB, a systematic diagnostic approach and further work-up should be done to exclude or confirm the diagnosis of the disease. Therefore, clinicians must be familiar with the clinical manifestations of these three diseases and should recognize the common and uncommon dermoscopic and histopathologic features of each. The misdiagnosis of these “the great imitators,” may lead to unfavorable outcomes.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent was not required as there are no patients in this study.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- A review of Leishmaniasis: Current knowledge and future directions. Curr Trop Med Rep. 2021;8:121-32.
- [CrossRef] [PubMed] [Google Scholar]
- Exclusive primary lesion of oral leishmaniasis with immunohistochemical diagnosis. Head Neck Pathol. 2016;10:533-7.
- [CrossRef] [PubMed] [Google Scholar]
- Cutaneous leishmaniasis: Recent developments in diagnosis and management. Am J Clin Dermatol. 2015;16:99-109.
- [CrossRef] [PubMed] [Google Scholar]
- Dermoscopy in the diagnosis of cutaneous leishmaniasis. Dermatol Pract Concept. 2019;9:111-8.
- [CrossRef] [PubMed] [Google Scholar]
- Cutaneous leishmaniasis: A great imitator. Clin Dermatol. 2020;38:140-51.
- [CrossRef] [PubMed] [Google Scholar]
- Cutaneous tuberculosis: A great imitator. Clin Dermatol. 2019;37:192-9.
- [CrossRef] [PubMed] [Google Scholar]
- Cutaneous granulomatosis: A comprehensive review. Clin Rev Allergy Immunol. 2018;54:131-46.
- [CrossRef] [PubMed] [Google Scholar]
- Cutaneous tuberculosis: Clinicopathologic arrays and diagnostic challenges. Dermatol Res Pract. 2018;2018:7201973.
- [CrossRef] [PubMed] [Google Scholar]
- Cutaneous and mucocutaneous leishmaniasis: Differential diagnosis, diagnosis, histopathology, and management. J Am Acad Dermatol. 2015;73:911-26, 927-8
- [CrossRef] [PubMed] [Google Scholar]
- Cutaneous tuberculosis overview and current treatment regimens. Tuberculosis (Edinb). 2015;95:629-38.
- [CrossRef] [PubMed] [Google Scholar]
- Unusual forms of cutaneous leishmaniasis due to Leishmania major. J Eur Acad Dermatol Venereol. 2016;30:1171-5.
- [CrossRef] [PubMed] [Google Scholar]
- Dermatoscopy of cutaneous granulomatous disorders. Indian Dermatol Online J. 2021;12:34-44.
- [CrossRef] [PubMed] [Google Scholar]
- Dermoscopy of sarcoidosis: A useful clue to diagnosis. Indian Dermatol Online J. 2018;9:80-1.
- [CrossRef] [PubMed] [Google Scholar]
- Cutaneous leishmaniasis: New dermoscopic findings. Int J Dermatol. 2013;52:831-7.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of dermoscopic features in cutaneous leishmaniasis. Al-Azhar Int Med J. 2023;4:178-84.
- [CrossRef] [Google Scholar]
- Dermoscopy of cutaneous granulomatous disorders: A study of 107 cases. Skin Res Technol. 2023;29:e13273.
- [CrossRef] [PubMed] [Google Scholar]
- Dermoscopy of tuberculosis verrucosa cutis. Indian Dermatol Online J. 2020;12:206-7.
- [CrossRef] [PubMed] [Google Scholar]
- Comparing dermatoscopic features with slit skin smear and histopathology in diagnosis of cutaneous leishmaniasis. Cureus. 2023;15:e35336.
- [CrossRef] [Google Scholar]
- Common features of tuberculosis and sarcoidosis. Int J Mycobacteriol. 2016;5(Suppl 1):S240-1.
- [CrossRef] [PubMed] [Google Scholar]
- Histopathology of cutaneous leishmaniasis caused by Leishmania donovani in Sri Lanka. Biomed Res Int. 2020;2020:4926819.
- [CrossRef] [PubMed] [Google Scholar]
- Cutaneous leishmaniasis: An evolving disease with ancient roots. Int J Dermatol. 2019;58:834-43.
- [CrossRef] [PubMed] [Google Scholar]
- A practical approach to cutaneous sarcoidosis. Am J Clin Dermatol. 2014;15:283-97.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical manifestations, diagnosis, and treatment of sarcoidosis. Mayo Clin Proc Innov Qual Outcomes. 2019;3:358-75.
- [CrossRef] [PubMed] [Google Scholar]
- Sarcoidosis and tuberculosis: The same disease with different manifestations or similar manifestations of different disorders. Curr Opin Pulm Med. 2012;18:506-16.
- [CrossRef] [PubMed] [Google Scholar]
- Cutaneous tuberculosis: Diagnosis, histopathology and treatment-part II. An Bras Dermatol. 2014;89:545-55.
- [CrossRef] [PubMed] [Google Scholar]
- Cutaneous leishmaniasis: Updates in diagnosis and management. Infect Dis Clin North Am. 2019;33:101-17.
- [CrossRef] [PubMed] [Google Scholar]
- Challenges in diagnosing Sarcoidosis in tuberculosis endemic regions: Clinical scenario in India. Sarcoidosis Vasc Diffuse Lung Dis. 2016;33:381-4.
- [Google Scholar]







