Translate this page into:
A study of serum cortisol levels in patients with lichen planus
*Corresponding author: Swaroopa Subhash, Department of Dermatology and Venereology, Government Medical College, Trivandrum, Kerala, India. swaroopasubhash2012@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Subhash S, Bindu RS, Nair PS, George AE. A study of serum cortisol levels in patients with lichen planus. J Skin Sex Transm Dis 2022;4:57-62.
Abstract
Objectives:
Primary objective was to compare between the serum cortisol levels in patients with lichen planus (LP) and normal subjects. The secondary objective was to compare between the serum cortisol levels of LP patients with and without oral lesions.
Materials and Methods:
This is a cross-sectional analytical study comparing the serum cortisol levels in patients with LP with that of age- and gender-matched normal subjects who attended a tertiary referral centre.
Results:
There were a total of 52 patients with LP in this study. The mean age was 48.1 ± 12.6 years. Male/female ratio was 1:1.9. Fifteen (28.8%) patients gave a history of recent stress in the 1 month before the onset of illness. Classical LP (34, 65.4%) was the most common type. Oral cavity was involved in 24 patients (46.2%). The mean serum cortisol level of patients with LP was higher than the same noted in age- and gender-matched normal subjects and this was statistically significant (P < 0.001). Statistically significant difference (P = 0.02) was noted between the serum cortisol levels of patients with different types of LP. No significant difference in the cortisol levels was observed between LP patients with and without oral lesions.
Limitations:
The main limitation of the study was the small sample size.
Conclusion:
This study showed a significant increase in serum cortisol levels in patients with LP when compared to normal subjects, while no significant difference was noted in cortisol levels between LP patients with and without oral lesions.
Keywords
Lichen planus
Cortisol
Stress
INTRODUCTION
Lichen planus (LP) is a chronic, mucocutaneous inflammatory disease with a prevalence of approximately 1%. Its etiology is not delineated completely. The various factors that can trigger the onset of disease include antigen-specific and non-specific mechanisms, emotional factors, and stress. Patients with LP have been found to have higher levels of stress as measured by various scales.[1] Stress can increase the serum cortisol levels.[2]
Cortisol is involved in energy, protein, and lipid metabolism, maintains vascular responsiveness, and affects the human stress response.[3] Cortisol has an impact on various pathological conditions including autoimmune and inflammatory diseases. There is evidence of neuroendocrine and immune dysregulation in patients with LP.[4] Very few studies have studied the role of stress in the onset and course of LP. Moreover, majority of prior studies are focused on patients with oral LP, with minimal or no cutaneous involvement. The severely pruritic lesions of LP can cause significant stress to the patient and this is more so in those with oral lesions since the latter may interfere with food intake. Hence, we conducted a study to compare between the serum cortisol levels in patients with LP and normal subjects.
MATERIALS AND METHODS
We designed a 1 year, hospital-based, cross-sectional analytical study in a tertiary care hospital, with the primary objective to compare between the serum cortisol levels in patients with LP and age- and gender-matched normal subjects. The secondary objective was to compare between the serum cortisol levels in LP patients with and without oral lesions. Patient and participant consents were obtained before enrolment in the study and approval was granted by the Institutional Ethics Committee of the hospital.
Study participants were grouped into Groups 1 and 2. Group 1 included consenting male and female LP patients who attended our hospital during the study period. Gender- and age-matched healthy males and females without any skin or mucosal lesions were included in Group 2. We excluded patients <12 years of age, patients who were receiving systemic steroids or oral contraceptive pills during the previous 1 month and pregnant females. Sample size was calculated based on the previous study conducted by Shetty et al.[5] A power analysis to calculate the sample size with alpha error of 0.05 and beta error of 20 and power of 80% was done. Assuming a mean difference in serum cortisol levels of 4 μg/dL between the two groups, the sample size required per group was 30 each in patient group and normal subjects.
We collected detailed history on demographics, comorbidities, duration of disease, and previous treatment using a structured questionnaire. To determine the stress experienced in the 1 month before the onset of LP, study participants were asked “Would you say you experienced a lot of stress, a moderate amount of stress, relatively little stress, or almost no stress at all?” Those who responded as having a lot of stress and moderate amount of stress were considered to have recent stress.[6] The degree of pruritus in skin lesions and degree of burning sensation in the oral cavity were rated by the patient on a numerical rating scale of 0–10 (mild -0–3, moderate - 4–7, and severe - 8–10).[6] Subsequently, a detailed dermatological clinical examination was done.
For serum cortisol estimation, 2 ml of blood was collected by venepuncture from each study participant, between 8 and 9 a.m. Blood was collected in a covered test tube and was allowed to clot. The clot was removed by centrifuging at 1000–2000 × g for 10 min in a refrigerated centrifuge. Serum was transferred into a clean polypropylene tube. The samples were then analyzed using the chemiluminescence method. Serum cortisol levels were expressed in μg/dL. Normal range of serum cortisol as per chemiluminescence method of estimation was 6.2–19.4 μg/dL.
Data collected were entered into Microsoft Excel spreadsheets and analyzed using trial version 18 of the statistical software SPSS. Quantitative variables were expressed as mean, standard deviation, and median. Qualitative variables were expressed as frequency and percentage. Serum cortisol levels of the study participants were expressed as mean with standard deviation. Mean serum cortisol level of patients with LP was compared with that of normal subjects using the unpaired Student’s “t” test. Similarly, mean serum cortisol levels of patients with LP with oral lesions and LP without oral lesions were compared using unpaired Student’s “t” test. One-way ANOVA test was used to compare between the mean serum cortisol levels of more than 2 groups. P ≤ 0.05 was considered as statistically significant.
RESULTS
A total of 52 patients (n = 52) with LP who attended our hospital and 52 normal age- and gender-matched subjects were included in the study, during the 1 year period. Age of the study population ranged between 15 and 77 years, with a mean age of 48.1 ± 12.6 years. Maximum number of patients were in the 51–60 years group (17/52, 32.7%). Among 52 patients, 34 (65.4%) were female and 18 (34.6%) were male with a male/female ratio of 1:1.9. Homemakers predominated in both patient (26, 50%) and control groups (21, 40.4%). Fifteen (28.8%) patients gave a history of recent stress in the 1 month before onset of illness. The major cause of stress was family issues followed by financial problems.
The clinical types of LP are given in Figure 1. Classical LP was the predominant clinical type (34, 65.4%). Oral cavity was involved in 24 patients (46.2%), with reticulate lesions being the most common (15/24, 62.5%). Twenty-four patients (46.2%) reported severe pruritus as per the grading mentioned in methods. Three patients (3/24, 12.5%) complained of severe burning sensation in the oral cavity.
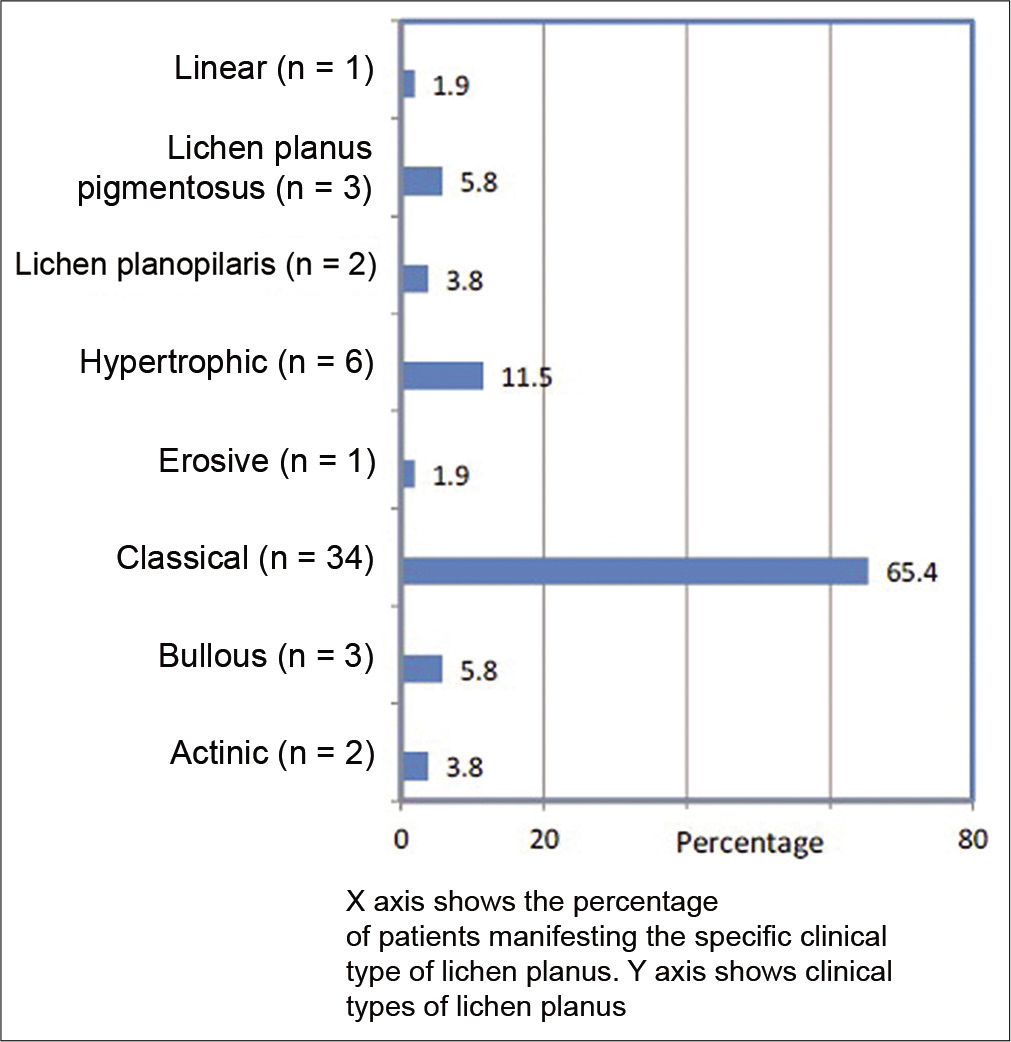
- Clinical types of lichen planus (n = 52).
Mean serum cortisol level of patients with LP was 10.1 ± 2.5 μg/dL and that of normal age- and gender-matched subjects was 8 ± 1.5 μg/dL [Figure 2]. The difference was statistically significant (P < 0.001).
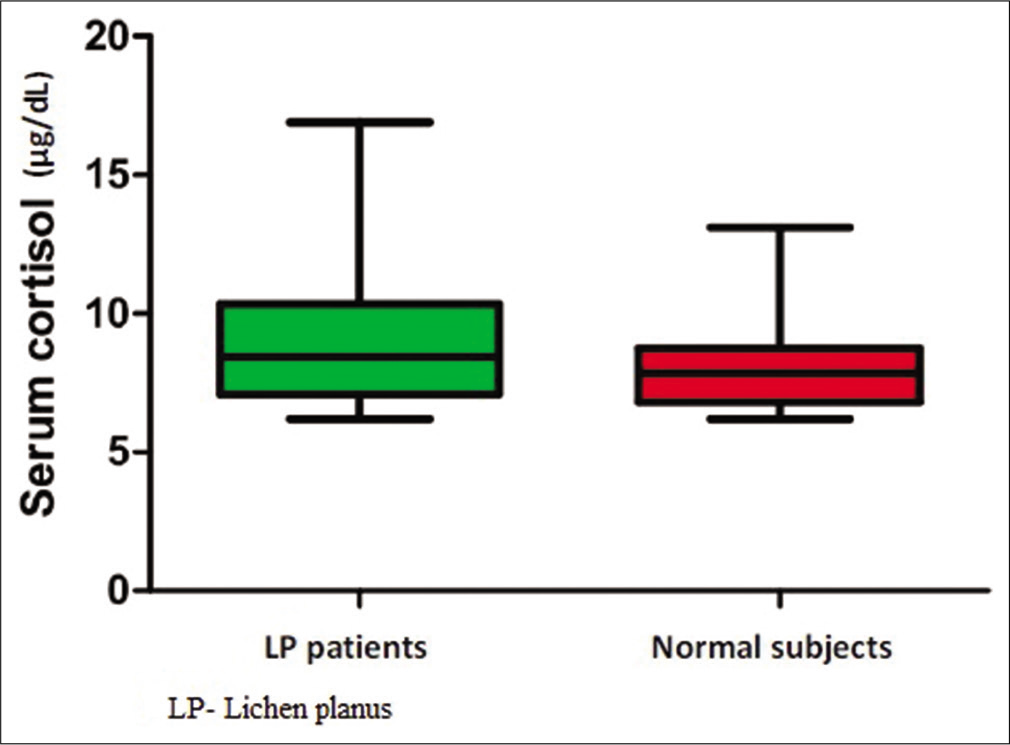
- Box plot showing serum cortisol levels in lichen planus patients and normal subject.
Among patients with LP, serum cortisol level ranged from 6.4 to 16.9 μg/dl. Among normal subjects, the serum cortisol level ranged from 6.2 to 13.1 μg/dl. Significant difference was observed between the mean serum cortisol levels of patients with different types of LP [Table 1] (P = 0.02). The highest mean serum cortisol value of 13.27 ± 0.51 μg/dl was seen in patients with bullous LP. The lowest mean serum cortisol level of 9.34 ± 1.87 μg/dl was seen in patients with classical LP [Table 1]. Among patients with LP, the mean serum cortisol level of males was 11.28 ± 2.23 μg/dl and that of females was 9.55 ± 2.72 μg/dl. The mean cortisol levels in males were higher than that in females and this was statistically significant (P = 0.02). Among normal subjects, the mean serum cortisol level of males was 8.29 ± 1.68 μg/dl and that of females was 7.37 ± 0.89 μg/dl and the gender difference among them was also significant (P = 0.03). There was no significant association (P = 0.89) between serum cortisol levels and age group [Table 2]. There was no significant association (P = 0.22) between serum cortisol levels and occupation [Table 3]. Association between serum cortisol and factors such as duration of disease, recent stress, severity of pruritus, and oral symptoms is given in Table 4.
| Types of LP | n | Serum cortisol (ug/dL) | ||
|---|---|---|---|---|
| Mean | Standard deviation | P-value | ||
| Actinic | 2 | 11.16 | 4.15 | 0.02 |
| Bullous | 3 | 13.27 | 0.51 | |
| Classical | 34 | 9.34 | 1.87 | |
| Erosive | 1 | 12.43 | 0 | |
| Hypertrophic | 6 | 12.40 | 3.41 | |
| Lichen planopilaris | 2 | 9.80 | 0.56 | |
| Lichen planus pigmentosus | 3 | 9.70 | 4.37 | |
| Linear | 1 | 12.40 | 0 | |
LP: Lichen planus, n: Number of patients
| Age group in years | Serum cortisol level in LP patients | Serum cortisol level in normal subjects | ||||||
|---|---|---|---|---|---|---|---|---|
| n | Mean (ug/dL) | SD | P-value | n | Mean (ug/dL) | SD | P-value | |
| <20 | 2 | 10.5 | 3.39 | 0.68 | 2 | 7.8 | 1.14 | 0.89 |
| 21–30 | 2 | 8.7 | 1.36 | 2 | 6.9 | 0.21 | ||
| 31–40 | 9 | 9.8 | 1.94 | 9 | 8.4 | 1.38 | ||
| 41–50 | 15 | 10.7 | 2.67 | 15 | 8.2 | 1.77 | ||
| 51–60 | 17 | 9.98 | 2.24 | 17 | 7.9 | 1.47 | ||
| >61 | 7 | 10.1 | 2.43 | 7 | 7.3 | 1.54 | ||
LP: Lichen planus, n: Number of patients, SD: Standard deviation
| Occupation | Serum cortisol (ug/dL) in LP patients (P=0.22) | Serum cortisol (ug/dL) in normal subjects (P=0.19) | ||||
|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | |
| Student | 3 | 10.27 | 3.62 | 3 | 7.57 | 0.91 |
| Homemaker | 26 | 9.63 | 2.27 | 21 | 8.34 | 1.90 |
| Manual laborer | 11 | 10.78 | 3.21 | 8 | 6.99 | 0.78 |
| Domestic help | 3 | 7.90 | 1.10 | 4 | 8.60 | 0.61 |
| Skilled work | 8 | 11.52 | 1.68 | 12 | 7.61 | 0.99 |
| Professional | 0 | 0 | 0 | 4 | 8.80 | 1.79 |
| Business | 1 | 12.00 | 0 | 0 | 0 | 0 |
LP: Lichen planus, n: Number of patients, SD: Standard deviation
| Disease factor | Disease factor subtype | n | Serum cortisol (ug/dL) | P-value | |
|---|---|---|---|---|---|
| Mean | SD | ||||
| Duration of illness | <1 month | 2 | 6.95 | 0.78 | 0.07 |
| >1 month | 50 | 10.23 | 2.49 | ||
| Recent stress | No | 37 | 10.17 | 2.50 | 0.91 |
| Yes | 15 | 10.08 | 2.67 | ||
| Severity of pruritus | Nil | 2 | 8.65 | 1.77 | 0.99 |
| Mild | 4 | 9.97 | 3.84 | ||
| Moderate | 22 | 9.62 | 2.40 | ||
| Severe | 24 | 10.78 | 2.44 | ||
| Burning sensation in oral cavity | No | 7 | 9.32 | 2.86 | 0.77 |
| Mild | 9 | 10.30 | 2.75 | ||
| Moderate | 5 | 10.53 | 2.31 | ||
| Severe | 3 | 9.07 | 1.99 | ||
| Type of oral LP | Erosive | 3 | 10.41 | 2.77 | 0.34 |
| Hyperpigmentation | 6 | 11.11 | 3.09 | ||
| Reticulate | 15 | 9.33 | 2.22 | ||
SD: Standard deviation, n: Number of patients, LP: Lichen planus
DISCUSSION
The present study included 52 cases of LP (n = 52) and equal number of age- and gender-matched normal subjects. The demographic and clinical features of this study were comparable with other similar studies in literature.[7] The female preponderance in this study was in accordance with literature as females are more prone to autoimmune diseases and LP being one. In this study, the mean serum cortisol of patients with LP was higher than the same in normal subjects and this was statistically significant (P < 0.001). Similar results were obtained by Shetty et al., Muhamood et al., and Chaitanya et al.[5,8,9] The pruritus and chronic skin lesions of LP contribute to stress and these, in turn, increase the serum cortisol levels. Other studies in literature have compared salivary cortisol levels of LP patients with the same in controls. A study by Tawil et al. and Nadendla et al. found statistically significant higher salivary cortisol levels in patients with LP in comparison to controls.[1,10] Studies by Lopez-Jornet et al. and Nosratzehi et al. also compared salivary cortisol levels between cases of oral LP and controls. They demonstrated increased levels in patients which were statistically significant.[11,12] Oral LP is associated with painful erosions and ulcers which may impair food intake. This, in turn, may contribute to increased stress and consequently elevated cortisol levels. However, in a study by Krasowska et al., there was no significant difference in the serum cortisol levels of patients with LP and healthy controls, and in a similar study, Girardi et al. also found no significant difference between the salivary cortisol levels of patients with oral LP and controls.[3,13] On the other hand, Pippi et al. found significantly decreased levels of salivary cortisol in patients with oral LP compared to controls.[14] In the present study, where serum cortisol levels were estimated, there was no statistically significant difference between the cortisol levels of LP patients with and without oral lesions (P = 0.99). There is a paucity of previous studies comparing the serum cortisol levels in LP patients with and without oral lesions. Even though we expect stress and cortisol levels to be elevated in any disease with a long duration like LP, in the present study, there was no significant association which was noted between the serum cortisol levels and the duration of the disease and this could be attributed to the paucity of patients who had a disease duration of less than one month (P = 0.07). Even though the cortisol levels were elevated in LP patients when compared to normal subjects, in the present study, no significant difference was noted between LP patients who had stress within the past 1 month and those who had no recent stress (P = 0.09). This is similar to the study conducted by Girardi et al.[13] However, other studies have shown significant relation between stress and LP.[15] This difference could be due to the inherent variability in self-reporting of stress and individual perception of stress.
The mean cortisol levels of males with LP were higher than females in this study and this was significant (P = 0.03). In normal subjects also, males had a higher mean cortisol level (P = 0.03). This could be explained by the fact that males could be more prone to stress related to their occupation and outdoor activity compared to females who were predominantly homemakers in this study. Similar findings were echoed in studies by Nadendla et al., and Koray et al.[10,16] A study by Sofer et al. also found higher serum-free cortisol in males when compared to females.[17]
However, in the present study, no significant difference was noted between cortisol levels and occupation either in the patient group (P = 0.22) or in the control group (P = 0.19). There was a statistically significant association between serum cortisol levels and clinical types of LP (P = 0.02). The maximum cortisol levels were seen in bullous LP followed by erosive and hypertrophic LP. This could be explained by the more symptomatic nature and the longer duration of disease associated with the aforementioned LP subtypes. Similar findings were demonstrated in the studies by Ivanovski et al. and Shah et al.[18,19] Jose et al. also found serum cortisol levels of patients with erosive LP to be significantly higher than that of controls and patients with non-erosive LP.[20] The mean serum cortisol levels of patients with LP did not show any significant variation with degree of pruritus (P = 0.99) or with burning sensation in the oral cavity (P = 0.77).
Limitations of the study
The main limitation of the study was the small sample size. However, the result of this study was concordant with most of the previous studies. We recommend further studies in larger number of patients with different clinical types of LP and different degrees of mucosal involvement. This can corroborate the role of cortisol in LP.
CONCLUSION
This study compared the serum cortisol levels of patients with LP and normal subjects belonging to the same age and gender and found a significant difference between the two groups (P < 0.001), with LP patients having much higher values than normal subjects. This result is concordant with most of the previous studies. However, this study could not find a significant difference between the serum cortisol levels of LP patients, with and without oral lesions (P = 0.99).
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
Dr. Pradeep S. Nair and Dr. Anuja Elizabeth George are on the editorial board of the Journal.
References
- Psychobiological aspects of patients with lichen planus. Curr Psychiatry. 2009;16:370-80.
- [Google Scholar]
- Lichen planus patients and stressful events. J Eur Acad Dermatol Venereol. 2008;22:437-41.
- [CrossRef] [PubMed] [Google Scholar]
- Psychological stress, endocrine and immune response in patients with lichen planus. Int J Dermatol. 2008;47:1126-34.
- [CrossRef] [PubMed] [Google Scholar]
- An association between serum cortisol levels in erosive and nonerosive oral lichen planus patients. Web Med Cent Dent. 2010;1:280-6.
- [Google Scholar]
- Assessment of depression in subjects with psoriasis vulgaris and lichen planus. J Eur Acad Dermatol Venereol. 2002;16:347-52.
- [CrossRef] [PubMed] [Google Scholar]
- Lichen planus: A clinical and epidemiological study. J Dermatol. 2000;27:576-82.
- [CrossRef] [PubMed] [Google Scholar]
- Estimation of serum cortisol levels in oral lichen planus patients and its relation to stress. Int J Contemp Med Res. 2018;2:856-60.
- [Google Scholar]
- Serological and psychological assessment of patients with oral lichen planus using serum cortisol levels and hads questionnaire-a case control study. J Popul Ther Clin Pharmacol. 2020;27:e19-27.
- [CrossRef] [PubMed] [Google Scholar]
- Association of salivary cortisol and anxiety levels in lichen planus patients. J Clin Diagn Res. 2014;8:ZC01-3.
- [CrossRef] [PubMed] [Google Scholar]
- Oral lichen planus: Salival biomarkers cortisol, immunoglobulin A, adiponectin. J Oral Pathol Med. 2016;45:211-7.
- [CrossRef] [PubMed] [Google Scholar]
- The evaluation of psychological factor and salivary cortisol and IgA levels in patients with oral lichen planus. Zahedan J Res Med Sci. 2014;16:31-4.
- [Google Scholar]
- Salivary cortisol and dehydroepiandrosterone (DHEA) levels, psychological factors in patients with oral lichen planus. Arch Oral Biol. 2011;56:864-8.
- [CrossRef] [PubMed] [Google Scholar]
- Diurnal trajectories of salivary cortisol, salivary α-amylase and psychological profiles in oral lichen planus patients. J Biol Regul Homeost Agents. 2014;28:147-56.
- [Google Scholar]
- Relation of stress and anxiety to oral lichen planus. Oral Surg Oral Med Oral Pathol. 1986;61:44-6.
- [CrossRef] [Google Scholar]
- The evaluation of anxiety and salivary cortisol levels in patients with oral lichen planus. Oral Dis. 2003;9:298-301.
- [CrossRef] [PubMed] [Google Scholar]
- Gender determines serum free cortisol: Higher levels in men. Endocr Pract. 2016;22:1415-21.
- [CrossRef] [PubMed] [Google Scholar]
- Psychological profile in oral lichen planus. J Clin Periodontol. 2005;32:1034-40.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of salivary cortisol and psychological factors in patients with oral lichen planus. Indian J Dent Res. 2009;20:288-91.
- [CrossRef] [PubMed] [Google Scholar]
- Estimation of serum cortisol levels in oral lichen planus patients with electrochemiluminescence. J Pharm Bioallied Sci. 2019;11:S265-8.
- [CrossRef] [PubMed] [Google Scholar]






