Translate this page into:
The use of topical rapamycin in successfully treating non-Langerhans cell histiocytosis in a pediatric patient
*Corresponding author: David Pudukadan, Department of Dermatology, Jubilee Mission Medical College and Research Institute, Thrissur, Kerala, India. davidpudukadan@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Pudukadan D, Jose JM. The use of topical rapamycin in successfully treating non-Langerhans cell histiocytosis in a pediatric patient. J Skin Sex Transm Dis 2024:6:44-6. doi: 10.25259/JSSTD_61_2023
Abstract
Non-Langerhans cell histiocytosis (non-LCH) refers to a collection of medical conditions distinguished by the excessive growth of histiocytes in bodily tissues. It is important to note that these conditions do not meet the established diagnostic criteria for Langerhans cell histiocytosis (LCH). Juvenile xanthogranuloma (JXG) and benign cephalic histiocytosis (BCH) represent the prevailing forms of cutaneous non-LCH. We present a case of JXG which responded to topical treatment with a 0.1% topical ointment of rapamycin. Rapamycin, an immunosuppressive and antineoplastic agent, can thus be a viable alternative non-invasive topical modality for managing JXG. However, it is imperative to conduct prolonged observations to evaluate the efficacy and potential adverse reactions.
Keywords
Rapamycin
Sirolimus
Juvenile xanthogranuloma
Histiocytosis
Pediatric
INTRODUCTION
Histiocytosis is a rare disorder that causes a build-up of macrophages, dendritic cells, or monocyte-derived cells in organs.[1,2] Juvenile Xanthogranuloma (JXG) presents with yellowish-red papules or nodules over the face, neck, and trunk.[2] These illnesses may have similar clinical and histological features. JXG is diagnosed by the presence of histiocytic infiltration and Touton giant cells.[3,4] JXG limited to the skin does not need therapy unless it is cosmetically disabling. In most patients (57.7%), the skin lesions tend to resolve completely, while in a few (6.6%), it resolves with a scar. Rapidly developing lesions with cosmetic issues may require treatment.[5,6] The available therapeutic modalities include low-dose radiation, intralesional steroid injection, CO2 laser, cryotherapy, and surgery as possible treatments.[1,2] To the best of our knowledge, only one case report using topical Rapamycin as a non-invasive therapeutic modality to treat JXG with favorable outcome is available. Inhibition of angiogenesis, and growth signal correction in numerous tumors make Rapamycin an immunosuppressive drug with an antineoplastic effect.[7] This article describes a case of JXG in a 2-year-old child treated with 1% topical rapamycin. Results were assessed based on photo documentation and lesion shrinkage.
CASE REPORT
A 7-year-old boy came to our dermatology clinic complaining of two painless and non-itchy orange-brown-colored plaques on the face and trunk. These lesions were first noticed when the child was three years old. The first lesion appeared on the lower back, and the second on the upper lip. There were no visible lesions at birth or a history of any trauma at the sites of these lesions. The family, personal, and medical history of the child were unremarkable. On examination, there was one brown-orange plaque on the face and one on the lower back. A dermoscopy examination of the face lesion showed a “setting sun appearance with a yellow hue,” as shown in Figure 1. The histopathological and immunohistochemistry examination of the lesion in the lower back revealed a non-centric granulomatous inflammation with Touton giant cells and was provisionally diagnosed as JXG. Immunohistochemistry was positive with CD68 and negative for CD1a and CD 117. The ophthalmology examination was unremarkable.
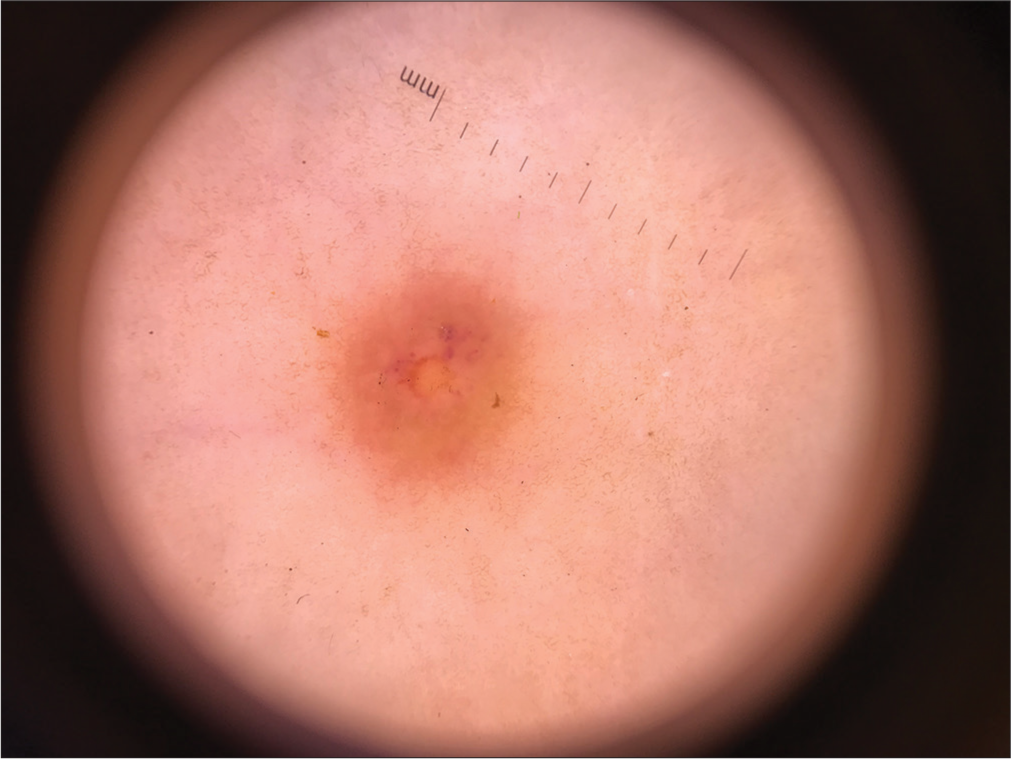
- The setting-sun pattern dermoscopy finding of juvenile xanthogranuloma papule (Dermlite 3, ×10).
The patient’s parents wanted a non-invasive treatment for the lesion on the face due to its visibility. Therefore, topical rapamycin therapy (30 tablets of 1 mg Sirolimus compounded in 30 g of lipophilic base to 0.1% ointment) was tried. It was applied once daily to the lip lesion, as this lesion was cosmetically disabling. After six weeks of treatment, the plaque on the upper lip flattened and faded. The size of this lesion decreased from 12 × 11 × 2 mm to 8 × 8 × 0.3 mm, as shown in Figures 2a and b.
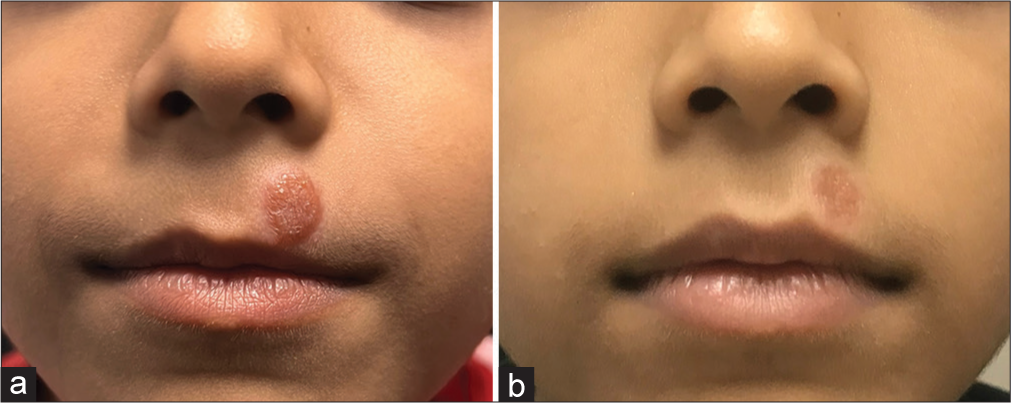
- Comparison of pre-treatment and post-treatment features in our case. (a): Tan-orange papules and plaques on the face before treatment. The child was treated with topical 1% rapamycin ointment twice daily for 16 weeks; (b): The skin lesion on the face flattened and faded and reduced in size.
DISCUSSION
The occurrence rate of JXG in children was 129 out of 24,600 pediatric tumor cases (0.5%) in a tumor registry spanning 35 years.[5] JXG is usually seen in young children with a median onset age of 2 years but may even be present at birth. A slight male preponderance was noted in various studies.[8] This article describes cutaneous lesions that began at the age of 36 months in a patient.
JXG affects the head and neck most commonly (44.2%), followed by the lower (21.8%), upper extremities (9.5%), and trunk (6.8%). Bilateral lesions can be seen in around 12.9%.[5] Extracutaneous JXG most commonly affects the eye, amounting to 0.3–10% of children with JXG and mostly in infants. Iris lesions are the most common presentation in the eye. The ocular complications include intraocular hemorrhage, glaucoma, and blindness.[9]
JXG restricted to the skin may not need any treatment, but surgical excision is sometimes done for cosmetic reasons.[2] In one study, 33 congenital JXG cases diagnosed at 0–14 months in New York were treated with a placebo, while the rest underwent excision (eight patients), intralesional (two patients), and systemic steroid (one patient).[10] Parents and doctors favor treatment of JXG with topical steroids, but it is rarely used as it can cause significant skin thinning.[11] As of the date of submission, to the best of our knowledge, only one rapamycin-treated JXG patient has been reported.[2] Our patient received 16 weeks of 1% topical rapamycin.
Streptomyces hygroscopicus fermented rapamycin inhibits mammalian target serine or threonine protein kinases (mTOR).[12] Its active ingredient inhibits angiogenesis and corrects tumor growth signals, making it an anti-cancer drug. Topical rapamycin for angiofibroma in tuberous sclerosis complex patients has been shown to work in several studies.[7,13,14] The pathophysiology of JXG is unknown. Based on our patient’s 1% topical rapamycin response, JXG may be related to the mTOR pathway.
Topical rapamycin for angiofibroma has been shown to work in several studies with varying vehicles, concentrations, and frequencies (0.003–1%).[7,13,14] Koenig et al.[15] studied the effectiveness and side effects of topical rapamycin in the treatment of angiofibroma in 59 patients. In this study, six patients (10.2%) reported discomfort at the site of application. It included itching (8.5%), acne (5.1%), redness (3.4%), and soreness (1.7%). Our patient did not show any adverse effects from the use of topical rapamycin.[11] Systemic rapamycin use can cause hypersensitivity, infection, and skin cancer.
In our case, we applied topical rapamycin once a day to the upper lip for 16 weeks. Since there is no objective measures to assess the progression of JXG, clinical photographic monitoring and lesion measurement were used to assess skin lesion improvements. The treated lesion showed a significant reduction in size. Our case report shows that rapamycin can be used if patients with JXG insist on topical treatment.
CONCLUSION
There is no proof to say for sure that 0.1% of rapamycin had worked in our case, as the lesions are known to have spontaneous resolution by eight years of age. However, the rapidity with which the lesions improved suggests that topical rapamycin may have worked and thus be used as a therapeutic modality for JXG. However, long-term effectiveness and side-effect monitoring are needed.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
Dr. David Pudukadan is on the editorial board of the Journal.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that they have used artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript or image creations.
Financial support and sponsorship
Nil.
References
- Histiocytosis In: Kang S, Amagai M, Bruckner AL, Enk AH, Margolis DJ, McMichael AJ, eds. Fitzpatrick’s dermatology (9th ed). New York, NY: McGraw-Hill Education; 2019. Available from: https://www.accessmedicine.mhmedical.com/content.aspx?aid=1161336567 [Last accessed on 2023 Nov 07]
- [Google Scholar]
- Successful treatment of non-langerhans cell histiocytosis with topical Rapamycin in two pediatric cases. Clin Cosmet Investig Dermatol. 2022;15:1575-82.
- [CrossRef] [PubMed] [Google Scholar]
- Benign cephalic histiocytosis: A case report. Ann Dermatol. 2011;23:508-11.
- [CrossRef] [PubMed] [Google Scholar]
- Non-infectious granuloma In: Practical dermatopathology. Houston, TX: Sanders; 2014. p. :115-6.
- [Google Scholar]
- Juvenile xanthogranulomas in Asian children. Dermatol Ther. 2022;35:e15224.
- [CrossRef] [PubMed] [Google Scholar]
- Rapid-growing juvenile xanthogranuloma on the scalp in 18-month-old girl. J Korean Neurosurg Soc. 2011;50:271.
- [CrossRef] [PubMed] [Google Scholar]
- Topical Rapamycin: A novel approach to facial angiofibromas in tuberous sclerosis. Arch Dermatol. 2010;146:715-8.
- [CrossRef] [PubMed] [Google Scholar]
- Juvenile xanthogranulomas in the first two decades of life: A clinicopathologic study of 174 cases with cutaneous and extracutaneous manifestations. Am J Surg Pathol. 2003;27:579-93.
- [CrossRef] [PubMed] [Google Scholar]
- Juvenile xanthogranuloma In: StatPearls. Treasure Island, FL: StatPearls Publishing; 2023. Available from: http://www.ncbi.nlm.nih.gov/books/NBK526103 [Last accessed on 2023 Dec 14]
- [Google Scholar]
- Congenital-type juvenile xanthogranuloma: A case series and literature review. Pediatr Dermatol. 2018;35:582-7.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment of benign cephalic histiocytosis with topical 1% rapamycin ointment. Pediatr Dermatol. 2019;36:411-3.
- [CrossRef] [PubMed] [Google Scholar]
- Topical rapamycin (sirolimus) for facial angiofibromas. Indian Dermatol Online J. 2013;4:54.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment of facial angiofibromas with topical application of oral rapamycin solution (1 mgmL (-1) in two patients with tuberous sclerosis. Br J Dermatol. 2011;165:922-3.
- [CrossRef] [PubMed] [Google Scholar]
- Topical sirolimus in the treatment of facial angiofibromas. Indian J Drugs Dermatol. 2018;4:49.
- [CrossRef] [Google Scholar]
- Efficacy and safety of topical Rapamycin in patients with facial angiofibromas secondary to tuberous sclerosis complex: The TREATMENT randomized clinical trial. JAMA Dermatol. 2018;154:773-80.
- [CrossRef] [PubMed] [Google Scholar]






